Structure and Dynamics of Macrophage Infectivity Potentiator Proteins from Pathogenic Bacteria and Protozoans Bound to Fluorinated Pipecolic Acid Inhibitors
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
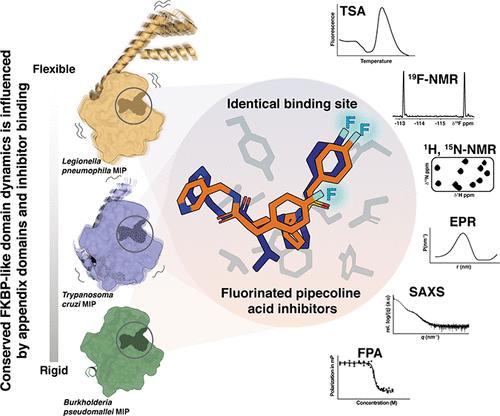
Macrophage infectivity potentiator (MIP) proteins, found in pro- and eukaryotic pathogens, influence microbial virulence, host cell infection, pathogen replication, and dissemination. MIPs share an FKBP (FK506 binding protein)-like prolyl-cis/trans-isomerase domain, making them attractive targets for inhibitor development. We determined high-resolution crystal structures of Burkholderia pseudomallei and Trypanosoma cruzi MIPs in complex with fluorinated pipecolic acid inhibitors. The inhibitor binding profiles in solution were compared across B. pseudomallei, T. cruzi, and Legionella pneumophila MIPs using 1H, 15N, and 19F NMR spectroscopy. Demonstrating the versatility of fluorinated ligands for characterizing inhibitor complexes, 19F NMR spectroscopy identified differences in ligand binding dynamics across MIPs. EPR spectroscopy and SAXS further revealed inhibitor-induced global structural changes in homodimeric L. pneumophila MIP. This study demonstrates the importance of integrating diverse methods to probe protein dynamics and provides a foundation for optimizing MIP-targeted inhibitors in this structurally conserved yet dynamically variable protein family.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


