Design, Synthesis, and Pharmacological Evaluation of Quinazoline and Quinoline Derivatives as Potent ENPP1 Inhibitors for Cancer Immunotherapy
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
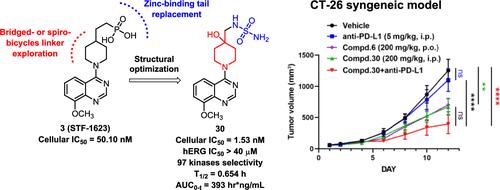
ENPP1, a transmembrane glycoprotein overexpressed in various cancers, has become a promising target for tumor immunotherapy. Several ENPP1 inhibitors have been reported, but only a few have been validated in vivo. Herein, based on the reported inhibitors 3 and 6, we carried out a structural optimization by designing a variety of 8-methoxyquinazoline and its equivalent 8-methoxy-3-cyano-quinoline derivatives featuring bridged- or spirobicycles as the linker. Compound 30 was identified as a promising ENPP1 inhibitor. This compound exhibited IC50 values of 8.05 nM against ENPP1 and 1.53 nM in MDA-MB-231 cells with no significant inhibitory effects against both hERG and a panel of 97 kinases. It effectively activated the intracellular STING pathway by inhibiting cGAMP degradation. In the murine CT-26 tumor model, 30 inhibited tumor growth with increased immune cell infiltration in the tumor microenvironment and enhanced type I interferon responses. Meanwhile, compound 30 synergically enhanced the antitumor efficacy of anti-PD-L1 antibody.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


