Coupling the oxyphilic metals and NbN to modulate the electronic structure of Pd and enhance the efficiency for alkaline MOR
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
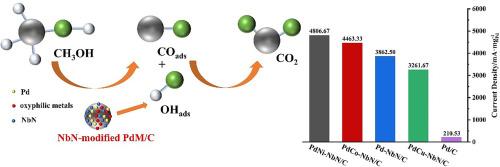
It is essential to design and produce stable, high-activity, and low-cost anode catalysts for direct methanol fuel cells (DMFCs). To improve the efficiency of the methanol oxidation reaction (MOR) in alkaline media, a series of PdM-NbN/C (M = Co, Ni, Cu) bi-component catalysts were synthesized by introducing various oxyphilic metals. Electrochemical evaluations show that the presence of Ni and Co significantly enhances catalytic activity, charge transfer kinetics, CO tolerance, and long-term stability compared to Pd-NbN/C. Among these, PdNi-NbN/C exhibits the highest mass activity of 4806.67 A·gPd−1, the lowest onset potential and charge transfer resistance, as determined from electrochemical testing. The rate-determining step (RDS) is identified as the COads oxidation reaction. Characterization analysis confirms that the second oxyphilic metals primarily optimize the Pd electronic structure and modulate the interaction between Pd and NbN, making a favorable shift in the d-band centers. Additionally, Ni and Co elements tend to form hydroxides in higher proportions, which aids in the removal of COads and accelerates the RDS. The electronic interactions between Pd, Ni, and NbN species are key to the enhanced catalytic performance for MOR. Pd nanoparticles in the electron-deficient condition may aid in the activation of methanol and its intermediates, as indicated by the linear relationship between the mass activities and d-band centers. This work offers important information for the logical design of efficient anode catalysts for DMFCs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


