Adsorption of Ni(II) from Aqueous Solution by Wheat Straw Modified with Mercaptopropionyl Functional Groups
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mercaptopropionyl wheat straw (MPWS) was prepared as an adsorbent by modifying wheat straw with mercaptopropionyl groups, and the ability of MPWS for the removal of Ni(II) from aqueous solution was examined. The removal of Ni(II) by using MPWS was identified through investigating the impacts of MPWS dosage, adsorption temperature, and adsorption time. Different models for the adsorption isotherm and kinetics were utilized to fit the experimental results and elucidate the mechanism of MPWS for Ni(II). Environmental interference factors, including initial Ni(II) concentration, pH value, inorganic matters, and organic matters in wastewater, were examined to evaluate the antienvironmental disturbance capability of MPWS during Ni(II) adsorption. A removal rate of Ni(II) as high as 99.02% was achieved at pH 6.0 with an adsorption temperature of 30 °C and a contact time of 100 min. The experimental results exhibited excellent alignment with both pseudo-second-order kinetic model, Freundlich isothermal model, Redlich–Peterson model, and Hill model. Furthermore, coexisting substances in the environment could inhibit the adsorption process of Ni(II) by MPWS; however, this inhibition could be mitigated or eliminated by increasing the amount of absorbent MPWS. Overall, MPWS displays remarkable resistance against environmental interference during its application for removing Ni(II) from wastewater.
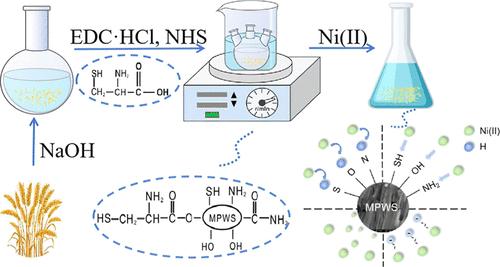
巯基丙酰修饰麦秸对Ni(II)的吸附研究
以巯基丙酸麦秸为吸附剂,对麦秸进行改性,制备巯基丙酸麦秸(MPWS),并考察其对Ni(II)的去除能力。通过考察MPWS用量、吸附温度和吸附时间对其去除Ni(II)的影响,确定了MPWS对Ni(II)的去除效果。利用不同的吸附等温线和动力学模型拟合实验结果,阐明MPWS对Ni(II)的吸附机理。考察了环境干扰因素,包括初始Ni(II)浓度、pH值、废水中无机物和有机物,以评价MPWS在吸附Ni(II)过程中的抗环境干扰能力。在pH为6.0、吸附温度为30℃、接触时间为100 min的条件下,对Ni(II)的去除率高达99.02%。实验结果与拟二级动力学模型、Freundlich等温模型、Redlich-Peterson模型和Hill模型均有很好的吻合。此外,环境中共存的物质会抑制MPWS对Ni(II)的吸附过程;然而,这种抑制可以通过增加吸附性MPWS的量来减轻或消除。总体而言,MPWS在去除废水中Ni(II)的过程中表现出显著的抗环境干扰能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


