Multilayer SiOx derived from Si–Ca alloy via Fe2O3 oxidization for Li-ion batteries
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
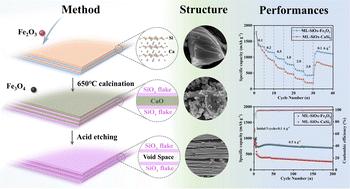
SiOx is deemed a promising candidate for lithium-ion batteries owing to its high specific capacity and relatively low volume expansion. However, its low rate performance is a bottleneck for its application. Two-dimensional SiOx with short lithium-ion pathways and large layer intervals has been a hot research topic for improving the electrochemical performance of lithium-ion batteries. Herein, a solid exfoliation method was designed to synthesize a multilayer SiOx using CaSi2 and Fe2O3. This multilayer SiOx exhibited large layer intervals after the by-products were removed by HCl. The void space provided extra space for volume expansion, which prevented pulverization, and the thin monolayer shortened the Li+ pathways. Therefore, ML-SiOx–Fe2O3 exhibited an excellent reversible capacity of 697.8 mA h g−1 after 200 cycles at 0.5 A g−1 with a capacity retention of 94.2%. Meanwhile, ML-SiOx–Fe2O3 anode delivered a rate performance of 432.7 mA h g−1 at 3 A g−1, and it could be recovered to 1157.1 mA h g−1 when the current density was converted to 0.1 A g−1. This work opens up a new method for synthesizing multilayer SiOx using metal oxides to exfoliate CaSi2.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


