Thermally Activated Delayed Fluorescence of Organic–Inorganic Hybrid Menshutkin-Type Cu–Sb Halides
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Organic–inorganic hybrid metal halides have attracted much attention due to their excellent optoelectronic properties. The heterometallic structure is expected to achieve more complex luminescence centers, thereby improving the tunability of the fluorescent materials. This paper reports the synthesis of crystals [Cu(2,2′-bpy)(PPh3)2][CuCl2(PPh3)2Sb2OCl4] (1, 2,2′-bpy = 2,2′-bipyridine, PPh3 = triphenylphosphine) and (Mim)2[Sb2OCl6] (2, Mim = 1-methylimidazole). Crystal 1 is a new Menshutkin-type compound with a double-layer hamburger-type structure, which is characterized by main group metal (lone pair)···π (arene) supramolecular interactions. Crystal 1 shows bright yellow light emission at room temperature and has thermally activated delayed fluorescence (TADF) characteristic with more than twice the increase of PL intensity from 170 to 320 K. The emission of crystal 1 arises from the charge transfer of the cation unit, as confirmed by the comparison study of crystals 1 and 2. Density functional theory (DFT) calculations further clarify that the enhanced PL performance and narrower bandgap are from the additional Cu(I) unit. Crystal 1 has good thermal stability and air stability, ensuring its anti-counterfeiting application. This work provides a new strategy for the construction of a Cu–Sb heterometallic compound.
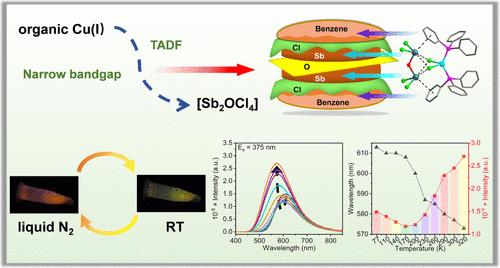
有机-无机杂化金属卤化物因其卓越的光电特性而备受关注。杂金属结构有望实现更复杂的发光中心,从而提高荧光材料的可调性。本文报道了[Cu(2,2′-bpy)(PPh3)2][CuCl2(PPh3)2Sb2OCl4](1,2,2′-bpy = 2,2′-联吡啶,PPh3 = 三苯基膦)和 (Mim)2[Sb2OCl6] (2,Mim = 1-甲基咪唑)晶体的合成。晶体 1 是一种新的门舒特金型化合物,具有双层汉堡包型结构,其特点是主族金属(孤对)--π(炔)超分子相互作用。晶体 1 在室温下发出明亮的黄色光,并具有热激活延迟荧光(TADF)特性,从 170 K 到 320 K,PL 强度增加了两倍多。密度泛函理论(DFT)计算进一步说明,增强的聚光性能和更窄的带隙来自附加的铜(I)单元。晶体 1 具有良好的热稳定性和空气稳定性,确保了其防伪应用。这项工作为构建铜锑异金属化合物提供了一种新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


