The Unexpected Role of Fluorine in the Band Gap Narrowing of Silver Niobium and Tantalum Pyrochlore Oxyfluorides
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
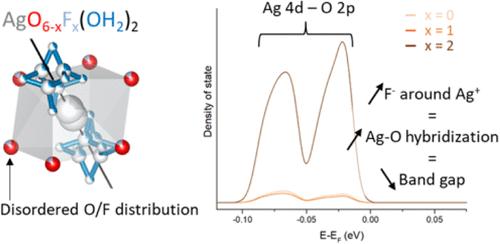
Mixed anion compounds have attracted growing interest in solid-state chemistry as a way to tailor physical properties. In this work, we synthesized new silver niobium and tantalum pyrochlore oxyfluorides by an ion-exchange reaction from Na2M2O5F2 (M = Nb or Ta). Instead of a classical Na+/Ag+ cation exchange, a less conventional dual cation and anion exchange reaction (2 Na+ + F–)/(Ag+ + H2O) takes place. Indeed, chemical and thermal analyses, as well as Rietveld refinement and 19F NMR, reveal the formation of AgTa2O5F·H2O and Na0.4Ag0.8Nb2O5F1.2·0.8H2O leading to a significant band gap narrowing of approximately 0.4 eV, as determined by diffuse reflectance spectroscopy. DFT calculations show that Ag 4d–O 2p states are located at the edge of the valence band and that the presence of fluorine in the coordination sphere of Ag promotes the hybridization and hence contributes to the band gap narrowing.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


