Sustained NF-κB activation allows mutant alveolar stem cells to co-opt a regeneration program for tumor initiation
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Disruptions to regulatory signals governing stem cell fate open the pathway to tumorigenesis. To determine how these programs become destabilized, we fate-map thousands of murine wild-type and KrasG12D-mutant alveolar type II (AT2) stem cells in vivo and find evidence for two independent AT2 subpopulations marked by distinct tumorigenic capacities. By combining clonal analyses with single-cell transcriptomics, we unveil striking parallels between lung regeneration and tumorigenesis that implicate Il1r1 as a common activator of AT2 reprogramming. We show that tumor evolution proceeds through the acquisition of lineage infidelity and reversible transitions between mutant states, which, in turn, modulate wild-type AT2 dynamics. Finally, we discover how sustained nuclear factor κB (NF-κB) activation sets tumorigenesis apart from regeneration, allowing mutant cells to subvert differentiation in favor of tumor growth.
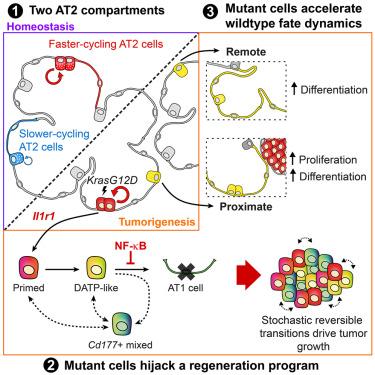
持续的NF-κB激活允许突变的肺泡干细胞参与肿瘤起始的再生程序
对控制干细胞命运的调节信号的破坏打开了肿瘤发生的途径。为了确定这些程序是如何变得不稳定的,我们在体内绘制了数千个小鼠野生型和krasg12d突变型肺泡II型(AT2)干细胞的命运图,并找到了两个独立的AT2亚群的证据,这些亚群具有不同的致瘤能力。通过结合克隆分析和单细胞转录组学,我们揭示了肺再生和肿瘤发生之间惊人的相似之处,暗示Il1r1是AT2重编程的共同激活因子。我们发现肿瘤的进化是通过获得谱系不忠和突变状态之间的可逆转换进行的,这反过来又调节了野生型AT2的动力学。最后,我们发现持续的核因子κB (NF-κB)激活如何将肿瘤发生与再生区分开来,使突变细胞破坏分化,有利于肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


