Characterization of tumour heterogeneity through segmentation-free representation learning on multiplexed imaging data
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
High-dimensional multiplexed imaging can reveal the spatial organization of tumour tissues at the molecular level. However, owing to the scale and information complexity of the imaging data, it is challenging to discover and thoroughly characterize the heterogeneity of tumour microenvironments. Here we show that self-supervised representation learning on data from imaging mass cytometry can be leveraged to distinguish morphological differences in tumour microenvironments and to precisely characterize distinct microenvironment signatures. We used self-supervised masked image modelling to train a vision transformer that directly takes high-dimensional multiplexed mass-cytometry images. In contrast with traditional spatial analyses relying on cellular segmentation, the vision transformer is segmentation-free, uses pixel-level information, and retains information on the local morphology and biomarker distribution. By applying the vision transformer to a lung-tumour dataset, we identified and validated a monocytic signature that is associated with poor prognosis. Self-supervised representation learning on data from imaging mass cytometry can be used to distinguish morphological differences in tumour microenvironments and to precisely characterize distinct microenvironment signatures.

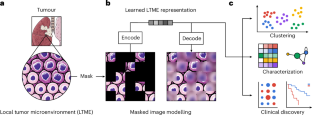
通过多路图像数据的无分割表示学习表征肿瘤异质性
高维多路成像可以在分子水平上揭示肿瘤组织的空间组织。然而,由于成像数据的规模和信息复杂性,发现和彻底表征肿瘤微环境的异质性是具有挑战性的。本研究表明,基于成像细胞术数据的自监督表示学习可以用来区分肿瘤微环境中的形态差异,并精确表征不同的微环境特征。我们使用自监督蒙面图像建模来训练一个视觉转换器,该转换器直接获取高维多路细胞术图像。与传统的依赖于细胞分割的空间分析相比,视觉转换器不需要分割,使用像素级信息,并保留局部形态和生物标记物分布的信息。通过将视觉转换器应用于肺肿瘤数据集,我们确定并验证了与预后不良相关的单核细胞特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


