Contextual computation by competitive protein dimerization networks
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
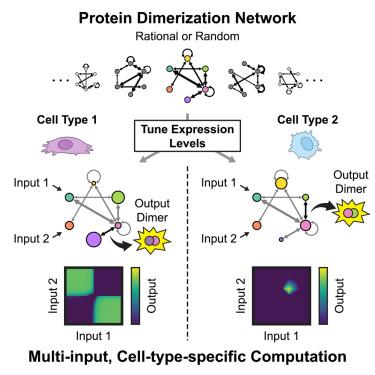
Many biological signaling pathways employ proteins that competitively dimerize in diverse combinations. These dimerization networks can perform biochemical computations in which the concentrations of monomer inputs determine the concentrations of dimer outputs. Despite their prevalence, little is known about the range of input-output computations that dimerization networks can perform and how it depends on network size and connectivity. Using a systematic computational approach, we demonstrate that even small dimerization networks of 3–6 monomers are expressive, performing diverse multi-input computations. Further, dimerization networks are versatile, performing different computations when their protein components are expressed at different levels, such as in different cell types. Remarkably, individual networks with random interaction affinities, when large enough, can perform nearly all potential one-input network computations merely by tuning their monomer expression levels. Thus, even the simple process of competitive dimerization provides a powerful architecture for multi-input, cell-type-specific signal processing.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


