PFOS causes lysosomes-regulated mitochondrial fission through TRPML1-VDAC1 and oligomerization of MCU/ATP5J2
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
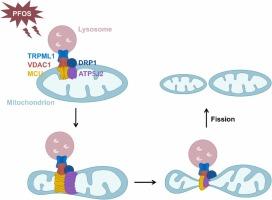
Perfluorooctane sulfonate (PFOS), a listed persistent organic pollutant, poses risks to human health and is closely linked to chronic metabolic diseases. Although the role of mitochondrial fission in these diseases has garnered attention, whether and how PFOS induces mitochondrial fission remains obscure. Here, we found that PFOS induced mitochondrial fission, as demonstrated by the fragmentation of mitochondria and the upregulation of dynamin-related protein 1 (DRP1), phospho-DRP1 and mitochondrial fission protein 1 (FIS1) in human hepatocytes MIHA and mice liver. Blocking the calcium transfer from lysosomes to mitochondria that was executed by transient receptor potential mucolipin 1 (TRPML1) of lysosomes and voltage-dependent anion channel 1 (VDAC1) of mitochondria, did not affect PFOS-induced mitochondrial fission. In contrast, knockdown of TRPML1 or VDAC1 reversed this process. Knockdown of mitochondrial calcium uniporter (MCU), rather than inhibiting its activity, effectively alleviated PFOS-induced mitochondrial fission. Additionally, PFOS increased MCU oligomers without affecting MCU monomer. Inhibiting autophagy reversed the MCU oligomerization. Further investigation unveiled the interactions of MCU with VDAC1, TRPML1, mitochondrial Fo complex subunit F2 (ATP5J2) and DRP1 in PFOS-exposed mice liver and MIHA cells. We also discovered that knockdown of ATP5J2 alleviated PFOS-induced mitochondrial fission. Ulteriorly, PFOS upregulated ATP5J2 that underwent oligomerization. Knockdown of MCU reversed the increase in ATP5J2. Our study uncovers the presence and molecular basics of lysosomes-regulated mitochondrial fission under PFOS exposure, explains the regulatory pathways on MCU and ATP5J2 oligomerization and their pivotal roles in mitochondrial fission, highlighting the involvement of mitochondrial fission in PFOS-related health risks.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


