Novel mechanisms for selenite biotransformation and selenium nanoparticles biogenesis in Acinetobacter sp. SX5 isolated from seleniferous soil
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
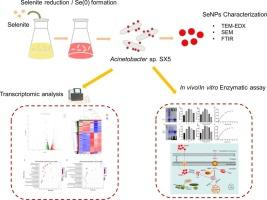
The high biotoxicity of selenium (Se) has spurred research into its microbial biotransformation into less toxic Se nanoparticles (SeNPs). However, the molecular mechanisms underlying microbially driven selenite transformation remain largely unknown. In the present study, Acinetobacter sp. SX5, a bacterial strain with high Se reduction capacity, was isolated from soil. The biotransformation of selenite by SX5 and the molecular mechanisms underlying the formation of SeNPs were investigated. SX5 almost completely transformed 5.0 mM selenite into intracellular and extracellular spherical SeNPs within 48 h. Fourier-transform infrared spectroscopy indicated that lipids, proteins, and carbohydrates were present on the surface of these SeNPs. Transcriptomic data subsequently revealed the significant upregulation of genes related to redox homeostasis and arsenate, pyruvate, and butanoate metabolism pathways. Gene mutation/complementation analysis confirmed that arsenate reductase (arsC) and NAD(P)-dependent alcohol dehydrogenase (dhaT1) facilitated selenite reduction in vivo. In vitro assays found that arsC and dhaT1 catalyzed Se(IV) reduction with NADPH acting as co-factor. To the best of our knowledge, this study is the first to present evidence for the participation of arsC and dhaT1 in selenite reduction in vivo, providing important insights into the molecular mechanisms underlying the biotransformation of Se(IV) and the biogenesis of SeNPs using Se-reducing bacteria.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


