Atomic Insights into the Heterogeneous Crystallization of Manganese (Oxyhydr)oxides on Typical Iron (Oxyhydr)oxides: from Adsorption to Oxidation to Crystallization
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Heterogeneous crystallization of manganese (oxyhydr)oxides (MnOx) on iron (oxyhydr)oxides (FeOx) is crucial for the biogeochemical cycling of Mn, yet atomic-level insights into this process are important but relatively limited. Herein, we revealed the distinct adsorption, oxidation, and crystallization mechanisms of Mn on hematite (Hem), ferrihydrite (Fhy), and goethite (Gth). Gth exhibited highest ability in Mn(II) removal and oxidation, followed by Hem and Fhy. Manganite and hausmannite were the main MnOx products with distinct proportions, and morphologies cross the systems. MnOx growth mechanisms involve surface-induced nucleation, crystallization by particle attachment (CPA), and self-catalyzed growth. On Fhy, self-catalyzed growth was dominant; for Gth, surface-induced nucleation was prevalent, supplemented by CPA; and Hem combined all three mechanisms. These distinct mechanisms led to nanoparticles primarily of hausmannite on Gth and nanowires of manganite and hausmannite on Hem and Fhy, with those on Hem displaying lower aspect ratios. Differences in MnOx structure and morphology were attributed to Mn(II)-FeOx complexation, FeOx electronic band structure, and crystal structure mismatch between MnOx and FeOx, which respectively influenced the direct and indirect electron transfer and heterogeneous nucleation efficiency. This work advances our understanding of MnOx crystallization on FeOx at the nanoscale, explaining the diverse morphology and structure of MnOx in different environments.
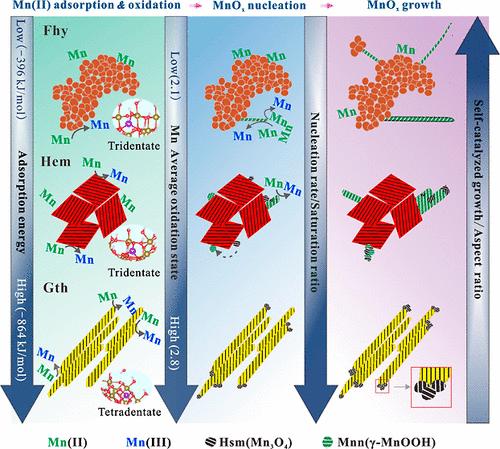
锰(氧合)氧化物在典型铁(氧合)氧化物上的非均相结晶:从吸附到氧化再到结晶
锰(氧)氧化物(MnOx)在铁(氧)氧化物(FeOx)上的非均相结晶对锰的生物地球化学循环至关重要,但对这一过程的原子水平的了解很重要,但相对有限。在此,我们揭示了Mn在赤铁矿(Hem)、水合铁(Fhy)和针铁矿(Gth)上不同的吸附、氧化和结晶机制。Gth对Mn(II)的去除和氧化能力最强,其次是Hem和Fhy。锰酸盐和锰酸盐是MnOx的主要产物,它们的比例不同,并且在不同的体系中具有不同的形态。MnOx的生长机制包括表面诱导成核、颗粒附着结晶(CPA)和自催化生长。在Fhy上,自催化生长占主导地位;Gth以表面诱导成核为主,辅之以CPA;而哼哼则结合了这三种机制。这些不同的机制导致Gth上主要是豪斯曼尼特的纳米颗粒,而Hem和Fhy上主要是锰矿和豪斯曼尼特的纳米线,而Hem上的纳米线显示出较低的纵横比。Mn(II)-FeOx络合作用、FeOx电子能带结构和MnOx与FeOx晶体结构的失配分别影响了MnOx与FeOx之间的直接和间接电子转移和非均相成核效率。这项工作促进了我们在纳米尺度上对氧化铁上MnOx结晶的理解,解释了不同环境下MnOx的不同形态和结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


