Divergent photoreduction of nitroaromatic compounds catalysed by dithienoquinoxaline
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
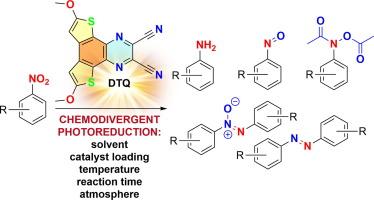
Nitroaromatic compounds can be conveniently reduced to anilines with the aid of a variety of chemical reductants. However, chemodivergent and chemoselective reduction of functionalized nitroaromatics into valuable nitroso, azo(xy), (hydr)azo and (hydroxyl)amino derivatives remains rather elusive. Electro- and photochemical strategies utilizing various catalytic systems and reaction media have been tested but most have been directed towards individual reduction products. Here, we present the photoredox-enabled chemodivergent reduction of nitroaromatics to nitroso, bis-(N,O-diacetyl)-N-arylhydroxylamine, azoxy, azo and amino derivatives. The developed protocol utilizes a novel photocatalyst, 6,9-dimethoxydithieno[2,3-f:3′,2′-h]quinoxaline-2,3-dicarbonitrile, and Hantzsch ester/triethanolamine as reductants. With the single organic photocatalyst, the reaction environment, temperature, time and catalyst loading can be varied to achieve chemodivergent photoreduction of nitroaromatics bearing various functional groups and to access a library of valuable products. The application of this methodology to multigram preparations of molecules of pharmaceutical and industrial relevance highlights its practical utility.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


