Psychological stress-induced local immune response to food antigens increases pain signaling across the gut in mice
IF 25.7
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
BACKGROUND & AIMS
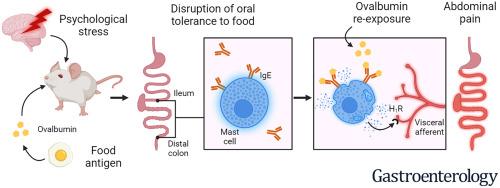
We recently showed that a bacterial infection can break oral tolerance to food and lead to IgE-dependent mast cell activation and food-induced abdominal pain, which could constitute an important pathogenic mechanism in post-infectious irritable bowel syndrome (IBS). Here, we investigated whether similar immune mechanisms in response to psychological stress lead to food-evoked pain signaling, and thus potentially explain the pathophysiology in a larger group of patients with IBS.METHODS
Mice were exposed to ovalbumin (OVA) during water avoidance stress (WAS) and re-exposed to OVA five weeks later. Nociception was evaluated by visceromotor responses and afferent nerve recordings to intestinal distension, and patch-clamp recordings of sensory neurons incubated with intestinal supernatants. The role of IgE and type 2 immunity was evaluated using pharmacological and genetic approaches.RESULTS
Re-exposure to OVA increased pain signaling in the colon and small intestine only in mice exposed to OVA during WAS, in the absence of systemic allergy. OVA-induced increases in pain responses depended on mast cells, IgE and STAT6 signaling. Notably, incubation of sensory neurons with ileum and colon supernatants from WAS/OVA+OVA mice lowered their threshold of excitability. Finally, treatment with histamine receptor H1 antagonist pyrilamine blocked the increased sensory neuron excitability, and reduced ileal afferent nerve firing to distension in WAS/OVA+OVA mice.CONCLUSIONS
Psychological stress induces a type 2 immune response to food antigens, with IgE-mediated mast cell activation and increased pain signaling in the small intestine and colon in response to food. These findings may explain the potential role of psychological stress in food-induced symptoms in IBS.
心理压力诱导的局部免疫反应对食物抗原增加疼痛信号在小鼠的肠道
背景,最近研究表明,细菌感染可破坏口腔对食物的耐受性,导致ige依赖性肥大细胞活化和食物性腹痛,这可能是感染后肠易激综合征(IBS)的重要致病机制。在这里,我们研究了类似的免疫机制对心理应激的反应是否会导致食物引起的疼痛信号,从而可能解释肠易激综合征患者的病理生理学。方法小鼠在避水应激(WAS)期间暴露于卵白蛋白(OVA), 5周后再次暴露于OVA。通过内脏运动反应和肠扩张的传入神经记录,以及肠上清孵育感觉神经元的膜片钳记录来评估伤害感受。采用药理学和遗传学方法评估IgE和2型免疫的作用。结果:在没有全身性过敏的情况下,仅在WAS期间暴露于OVA的小鼠中,再次暴露于OVA增加了结肠和小肠中的疼痛信号。ova诱导的疼痛反应增加依赖于肥大细胞、IgE和STAT6信号传导。值得注意的是,用WAS/OVA+OVA小鼠的回肠和结肠上清液孵育感觉神经元降低了它们的兴奋性阈值。最后,用组胺受体H1拮抗剂吡拉胺治疗WAS/OVA+OVA小鼠,阻断了感觉神经元兴奋性的增加,并减少了回肠传入神经的放电至扩张。结论心理应激诱导对食物抗原的2型免疫反应,通过ige介导的肥大细胞活化和小肠和结肠疼痛信号的增加对食物做出反应。这些发现可以解释心理压力在肠易激综合征食物诱发症状中的潜在作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gastroenterology
医学-胃肠肝病学
CiteScore
45.60
自引率
2.40%
发文量
4366
审稿时长
26 days
期刊介绍:
Gastroenterology is the most prominent journal in the field of gastrointestinal disease. It is the flagship journal of the American Gastroenterological Association and delivers authoritative coverage of clinical, translational, and basic studies of all aspects of the digestive system, including the liver and pancreas, as well as nutrition.
Some regular features of Gastroenterology include original research studies by leading authorities, comprehensive reviews and perspectives on important topics in adult and pediatric gastroenterology and hepatology. The journal also includes features such as editorials, correspondence, and commentaries, as well as special sections like "Mentoring, Education and Training Corner," "Diversity, Equity and Inclusion in GI," "Gastro Digest," "Gastro Curbside Consult," and "Gastro Grand Rounds."
Gastroenterology also provides digital media materials such as videos and "GI Rapid Reel" animations. It is abstracted and indexed in various databases including Scopus, Biological Abstracts, Current Contents, Embase, Nutrition Abstracts, Chemical Abstracts, Current Awareness in Biological Sciences, PubMed/Medline, and the Science Citation Index.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


