Medicinal chemistry applications of the Dimroth Rearrangement to the synthesis of biologically active compounds
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
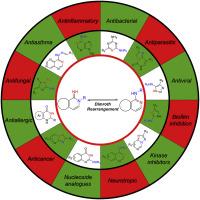
The Dimroth Rearrangement (DR) is an isomerization process involving the translocation of exo- and endocyclic nitrogen atoms in heterocyclic systems via a ring opening, rotation, and ring closure mechanism. Originally discovered over 120 years ago, the mechanistic occurrence of the DR on multiple heterocycles has been widely studied, and its application to the synthesis of biologically active compounds is well documented, albeit on some occasions not directly referenced. A surprisingly high number of drug discovery programs take advantage of the DR for the synthesis of heterocycle-containing compounds, including 4-aminopyrimidines and 4-anilinoquinazolines. Evidence of the flexibility and valuable potential of the DR can be found in the use of this reaction in the manufacture processes of several active pharmaceutical ingredients (APIs) on a commercial scale, allowing a reduction in the manufacturing costs and the environmental burden of the synthetic routes. The aim of this review is to outline the generality and broad applicability of the DR to the synthesis of biologically active compounds and highlight the opportunities to utilize this tool more widely within the medicinal chemistry toolbox.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


