Gradient-Modulated Synthesis of Dense, Uniform Cu@Ag: Low Silver, High Conductivity, and Oxidation Resistant
IF 5.8
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
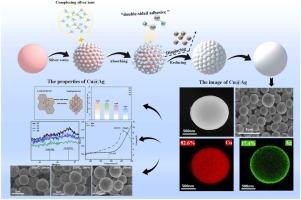
The pursuit of Cu@Ag composites stems from a strategic balance between cost and performance. Copper powder, inexpensive but prone to oxidation, necessitates silver coating to bolster oxidation resistance and conductivity. Low silver content Cu@Ag composites present a formidable challenge, requiring innovative methods and routes for uniform and dense coating. Moreover, the intricate correlations between Cu@Ag's electrical conductivity and its microstructure remains elusive, necessitating in-depth investigations to unravel the structure-property relationships and optimize performance. Herein, we successfully develop a facile gradient-modulated method to prepare Cu@Ag with a thin but dense coating layer. The Gradient-Modulated Synthesis of Cu@Ag involves initially regulating seed-induced growth and silver layer formation through the gradient addition of silver sources and complexing agents. Subsequently, dynamic control over the grain size of the silver layer is achieved via the gradient addition of reducing agents. Two complexing agents are employed, sodium tartrate as the first complexing agent, which enables a layer of silver seed to grow on the surface of the copper first. Next L-histidine is used as the second complexing agent, playing a role of a “double-sided adhesive” capable of forming link with both silver and sliver ion. Silver ions have been transferred onto the silver seed on the copper surface, then simultaneously displaced and reduced them to complete the coating. The result shows that the synthesized Cu@Ag can achieve a complete and uniform silver layer (29 nm) under a low silver content (17.4%). In addition, the Cu@Ag powder exhibited high oxidative stability, thermal stability, good resistance to solid state dehumidification and extremely low resistivity (10.642 µΩ·cm). Furthermore, we can effectively tailor the grain size by meticulously controlling the reduction and displacement durations during the synthesis process. Results indicated that the conductivity of silver-coated copper powder is intricately linked to its grain size.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


