Isomeric 2D and 3D covalent organic frameworks induced by inter-modular hydrogen bonds for photocatalytic CO2 reduction
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
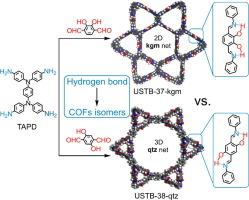
The rational adjustment of conformational polymorphism of building blocks is able to generate isomeric extending frameworks with different two- and three-dimensional (2D and 3D) structures, being available but very challenging. Herein, the hydrogen-bonding locking strategy is proposed to tune the molecular conformations of building block for crystallizing 2D and 3D covalent organic frameworks (COFs). Under the same reaction conditions, the imine condensation of N,N,N′,N′-tetrakis(4-aminophenyl)-1,4-phenylenediamine (TAPD) and dihydroxyterephthalaldehyde isomers generates isomeric COFs with 2D kgm and 3D qtz topology, respectively. Their isomeric structures have been disclosed by investigations of powder X-ray diffraction in combination with high resolution transmission electron microscopy. This is due to that the distinct inter-modular hydrogen bonds induce the conformation change of TAPD. Post-metalation of COFs isomers with either catechol or 2-(benzylideneamino)phenol sites are conducted for the photocatalysis of CO2 reduction. The cobalt-containing COFs show the CO production rate of up to 7575 µmol g−1h−1 and selectivity of 94 %. This work opens a new gate by introducing hydrogen bonding moieties to simultaneously adjust frameworks structures and functionalities.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


