Using mathematical modelling and AI to improve delivery and efficacy of therapies in cancer
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
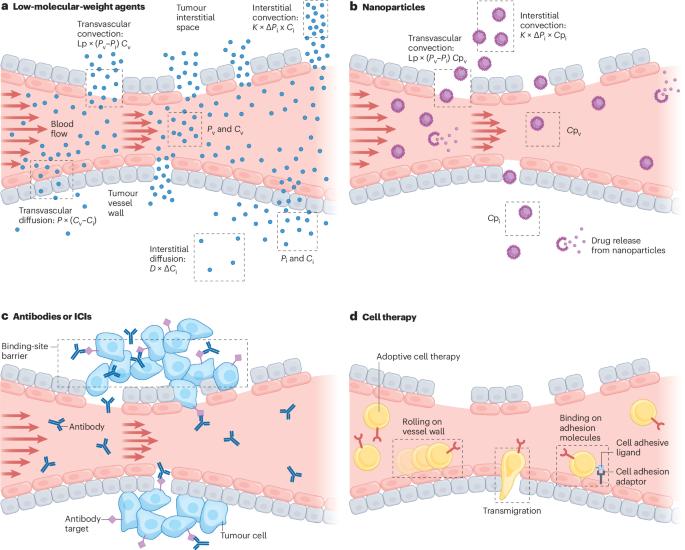
Mathematical modelling has proven to be a valuable tool in predicting the delivery and efficacy of molecular, antibody-based, nano and cellular therapy in solid tumours. Mathematical models based on our understanding of the biological processes at subcellular, cellular and tissue level are known as mechanistic models that, in turn, are divided into continuous and discrete models. Continuous models are further divided into lumped parameter models — for describing the temporal distribution of medicine in tumours and normal organs — and distributed parameter models — for studying the spatiotemporal distribution of therapy in tumours. Discrete models capture interactions at the cellular and subcellular levels. Collectively, these models are useful for optimizing the delivery and efficacy of molecular, nanoscale and cellular therapy in tumours by incorporating the biological characteristics of tumours, the physicochemical properties of drugs, the interactions among drugs, cancer cells and various components of the tumour microenvironment, and for enabling patient-specific predictions when combined with medical imaging. Artificial intelligence-based methods, such as machine learning, have ushered in a new era in oncology. These data-driven approaches complement mechanistic models and have immense potential for improving cancer detection, treatment and drug discovery. Here we review these diverse approaches and suggest ways to combine mechanistic and artificial intelligence-based models to further improve patient treatment outcomes. Here Harkos et al. review the role of continuous models and discrete models in predicting and understanding therapy delivery and efficacy in solid tumours. They propose ways to integrate mechanistic and AI-based models to further improve patient outcomes.
利用数学建模和人工智能来改善癌症治疗的递送和疗效
数学模型已被证明是预测实体肿瘤分子、抗体、纳米和细胞治疗的递送和疗效的宝贵工具。基于我们对亚细胞、细胞和组织水平的生物过程的理解的数学模型被称为机械模型,而机械模型又被分为连续模型和离散模型。连续模型进一步分为集总参数模型-用于描述肿瘤和正常器官中药物的时间分布-和分布参数模型-用于研究肿瘤中治疗的时空分布。离散模型捕获细胞和亚细胞水平上的相互作用。总的来说,这些模型通过结合肿瘤的生物学特性、药物的物理化学特性、药物、癌细胞和肿瘤微环境的各种组成部分之间的相互作用,以及结合医学成像实现患者特异性预测,有助于优化肿瘤分子、纳米级和细胞治疗的递送和疗效。基于人工智能的方法,如机器学习,开创了肿瘤学的新时代。这些数据驱动的方法补充了机制模型,在改善癌症检测、治疗和药物发现方面具有巨大的潜力。在这里,我们回顾了这些不同的方法,并提出了结合机械和基于人工智能的模型来进一步改善患者治疗结果的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


