Ultrafast Formation of Microdroplet Arrays with Chemical Gradients for Label-Free Determination of Enzymatic Reaction Kinetics
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
High throughput assays including enzymatic reactions are usually conducted in multiwell plates and analyzed in plate readers. This approach has limitations in i) upscaling and ii) the choice of reactions, as labeled compounds are required. A technique is introduced to rapidly generate dense microdroplet arrays by stream shearing (MASS). The fluid is delivered via a capillary onto a glass plate that carries a pattern of hydrophilic spots surrounded by a hydrophobic coating. Moving the glass plate shears off droplets from the fluid stream that are retained on the hydrophilic spot. Arrays of up to 24 192 homogenous droplets (coefficient of variation: 2.9 %) with defined volumes from 280 to 980 pL are generated in less than 22 min. Thereby, the droplet content is varied, and fine chemical gradients are obtained across the plate, which are analyzed with both fluorescence microscopy and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). This method is employed for label-free kinetic studies of an enzymatic reaction. For the cleavage of angiotensin II-fluorescein by the angiotensin II-converting enzyme, a maximum reaction velocity (vmax) of 5.7 µm min−1 is determined and the Michaelis-Menten constant (KM) of 84.8 µm is found. The platform can be further upscaled for biochemical assays as required for drug discovery and protein engineering.
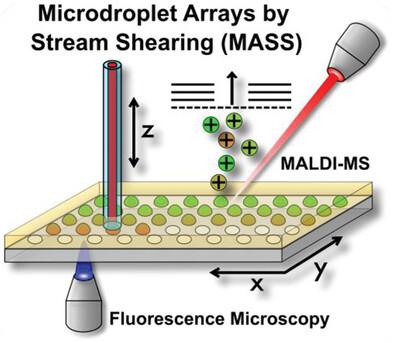
包括酶反应在内的高通量检测通常在多孔板中进行,并在平板阅读器中进行分析。由于需要标记化合物,这种方法在 i) 放大和 ii) 反应选择方面存在局限性。本文介绍了一种通过流剪切(MASS)快速生成致密微滴阵列的技术。流体通过毛细管输送到玻璃板上,玻璃板上有亲水点图案,周围有疏水涂层。移动玻璃板可剪切掉流体中保留在亲水点上的液滴。在不到 22 分钟的时间内,就能产生多达 24 192 个均匀液滴(变异系数:2.9%)的阵列,液滴体积从 280 pL 到 980 pL 不等。这样,液滴的含量就会发生变化,并在整个平板上获得精细的化学梯度,然后用荧光显微镜和基质辅助激光解吸电离质谱(MALDI-MS)对其进行分析。这种方法可用于酶反应的无标记动力学研究。在血管紧张素 II 转化酶裂解血管紧张素 II-荧光素的过程中,确定了最大反应速度(vmax)为 5.7 µm min-1,并发现迈克尔斯-门顿常数(KM)为 84.8 µm。该平台可进一步升级,用于药物发现和蛋白质工程所需的生化测定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


