Unmasking the UV Photobleaching of β-Diketonate [Eu(BTFA)4]− Complexes as an Energy-Driven Photoreduction Process
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
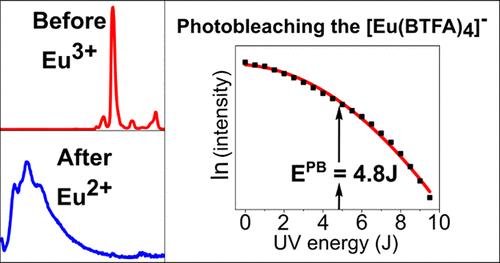
We elucidate the nature of the gradual attenuation of luminescence under sustained ultraviolet (UV) radiation exposure in europium [Eu(BTFA)4]− complexes, revealing that it originates from a nonlinear energy-driven process, wherein Eu(III) is irreversibly reduced to Eu(II). This photoreduction was confirmed via the electrochemical techniques cyclic voltammetry and chronoamperometry, which, coupled with luminescence experiments, provided direct evidence of the Eu3+/Eu2+ reduction and the disappearance of Eu(III) after UV exposure. Indeed, extended UV exposure induces a gradual decrease in Eu(III) luminescence, paired with an increased intensity of Eu(II) luminescence, upon direct excitation of the metal ion. To provide an adequate description of this process, we advanced a photochemical law in the energy domain (rather than the time domain) and introduced the concept of photobleaching energy to facilitate comparisons of photostability among various complexes in different solvents. The nonlinearities detected in the energy domain suggest an underlying multiphoton process. As a consequence, we uncovered a trade-off between photostability and luminescence: enhancing one implies diminishing the other. Specifically, the proximity between the complex and cation in nonpolar solvents induces asymmetry in the complex, enhancing luminescence but reducing photostability. Conversely, distancing the cation leaves the complex within a more symmetrical solvent enclosure, decreasing luminescence and increasing photostability. Our findings also indicate that exposing Eu(III) complexes to UV light can function as a method for the photochemical synthesis of Eu(II) complexes, a process further substantiated by our electrochemical experiments.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


