Lanthanide Oxalates: From Single Crystals to 2D Functional Honeycomb Nanosheets
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
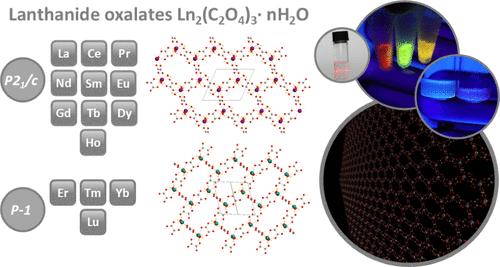
Oxalates are simple, low-cost but crucial compounds in the technology of lanthanides, actinides, and transition metals. Apart from using oxalate as a versatile ligand in coordination chemistry, simple oxalate salts are still under a scientific focus, linked with ion batteries, optical and magnetic materials, and, most importantly, industrial-technological mining and separation loops. The typically low solubility of oxalate salts is advantageous from the viewpoint of a convenient and affordable synthesis requiring only green solvents. Even though basic lanthanide oxalates have been known for decades, their structural descriptions have remained fuzzy, especially concerning water content and heavy lanthanide analogues. Herein, we present a newly developed preparation technique for large oxalate monocrystals applied to the whole lanthanide series. All of the structures were reviewed, and some new structures were determined. All of these oxalates exhibit a honeycomb structure with closed cavities containing water molecules. These honeycomb coordination polymers form a layered structure bonded by hydrogen bonds. Surprisingly, most oxalates can be easily exfoliated/delaminated in EtOH, forming colloids of up to single-layered nanosheets. Such a feature has never been described for 2D lanthanide oxalates and demonstrates a new form of applicability for them, e.g., for the construction of thin films or inkjet-printed layers using an extremely facile and economical preparation route.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


