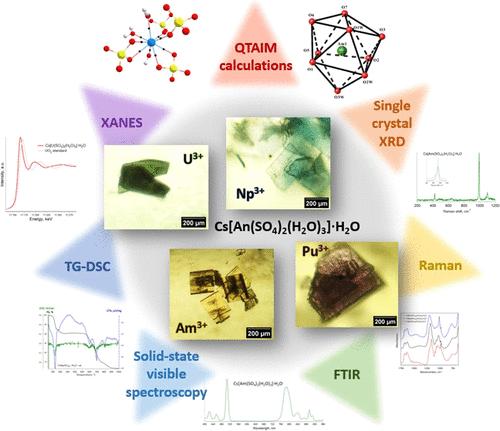
Structural Regularities, Thermal Stability, and Nature of Chemical Bonding in the Series of Actinide Double Sulfates Cs[An(SO4)2(H2O)3]·H2O (An = U, Np, Pu, or Am)
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Investigation of the properties of the trivalent light actinide compounds is hindered by their low stability under normal conditions. In this study, the An3+ double sulfates Cs[An(SO4)2(H2O)3]·H2O (An = U, Np, Pu, or Am) were synthesized and characterized by complementary methods. Their structure was solved using single-crystal X-ray diffraction (XRD), and peculiarities of the sulfate anion environment were addressed with vibrational spectroscopy. The oxidation states of the actinides were confirmed by using X-ray absorption near edge spectroscopy (XANES) and solid-state absorption spectroscopy. Changes in the local environment of Am ions caused by self-irradiation are observed after several months of storage. Decomposition of the compounds in air and in the inert atmosphere at temperatures up to 1000 °C and the final products were studied using thermal analysis and powder diffraction. Computational investigation employing approaches such as QTAIM, Löwdin bond order analysis, and atomic charge calculations was used to investigate trends in the nature of chemical bonds in these compounds. It is shown that the covalent interaction decreases from U to Am with a corresponding increase in ion charge.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


