Bifunctional Synergistic Mg@SnSb SEI for Low Interfacial Reaction Energy Barriers and Stable Cycling of High-Performance Rechargeable Magnesium Batteries
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The formation of a stable passivation layer and the strong electrostatic interactions impede the diffusion of magnesium ions (Mg2+) at the Mg anode surface. Construction of an artificial solid electrolyte interphase (SEI) layer presents a promising approach to overcome these limitations. This study develops a synergistic and structurally stable Mg@SnSb SEI through an in situ reaction between the anode and a Tin trifluoromethanesulfonate and antimony chloride (Sn(OTf)2-SbCl3-based) electrolyte, featuring a low LUMO (lowest unoccupied molecular orbital). The in situ formed multi-phase SEI effectively reduces the interfacial reaction barriers and facilitates Mg2+ diffusion during both the plating and the stripping processes. Additionally, the formation of nano-grained microstructure enhances the uniformity of Mg plating/stripping and suppresses the decomposition of the OTf anions and DME solvent molecules. The Mg anode incorporating the Mg@SnSb SEI exhibits an exceptionally low overpotential of less than 0.07 V and an ultra-long cycle life exceeding 1500 h. In full-cell tests using Mg@SnSb||Mo6S8, the system achieved exceptional electrochemical performance, maintaining over 94% of its initial capacity after more than 400 cycles.
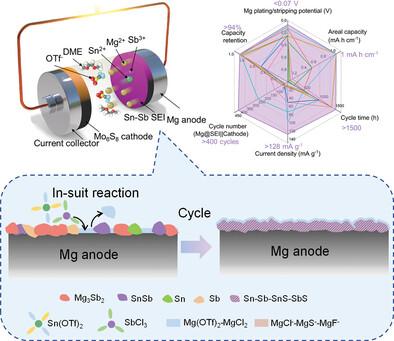
低界面反应能垒和高性能可充电镁电池稳定循环的双功能协同Mg@SnSb SEI
钝化层的形成和强静电相互作用阻碍了镁离子(Mg2+)在镁阳极表面的扩散。人工固体电解质间相(SEI)层的构建为克服这些限制提供了一种很有希望的方法。本研究通过阳极与三氟甲烷磺酸锡和氯化锑(Sn(OTf)2- sbcl3基)电解质的原位反应,开发了一种具有低LUMO(最低未占据分子轨道)的协同且结构稳定的Mg@SnSb SEI。原位形成的多相SEI有效地降低了界面反应障碍,促进了镀和剥离过程中Mg2+的扩散。此外,纳米颗粒结构的形成提高了镀/剥离Mg的均匀性,抑制了OTf阴离子和二甲醚溶剂分子的分解。含有Mg@SnSb SEI的Mg阳极具有低于0.07 V的极低过电位和超过1500小时的超长循环寿命。在使用Mg@SnSb||Mo6S8的全电池测试中,该系统取得了卓越的电化学性能,在400多次循环后保持了超过94%的初始容量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


