One-Pot Synthesis of Novel Tetrasubstituted α-Aminophosphonates Derived from α-Methylphosphoserine and In Vivo Evaluation as Anti-Inflammatory Agents
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
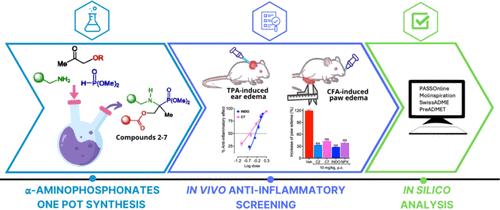
A series of new tetrasubstituted α-aminophosphonate derivatives with a methylphosphoserine fragment were described. These compounds were synthesized by a three-component (3-CR) “Kabachnik-Fields reaction.” The novel α-aminophosphonates were screened for in vivo anti-inflammatory activity through topical and oral administration routes. All compounds decreased TPA-induced ear edema in a dose-dependent fashion. In this test, compounds 2, 5, and 7 showed the same efficacy (≈ 90%) and higher potency than indomethacin and decreased the inflammatory marker neutrophil-to-lymphocyte ratio (NLR). Moreover, oral pretreatment and post-treatment with compounds 2–7 reduced CFA-induced paw edema, as did indomethacin or (S)-naproxen. Based on the promising in vivo anti-inflammatory results, we investigated their physicochemical and pharmacokinetics profiles in silico. The analysis also revealed that the novel tetrasubstituted α-aminophosphonates did not break Lipinski’s rule of five and had drug-likeness and favorable ADME properties for oral and transdermal administration.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


