Microfluidic 3D Bioprinting of Foamed Fibers with Controlled Micromorphology
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
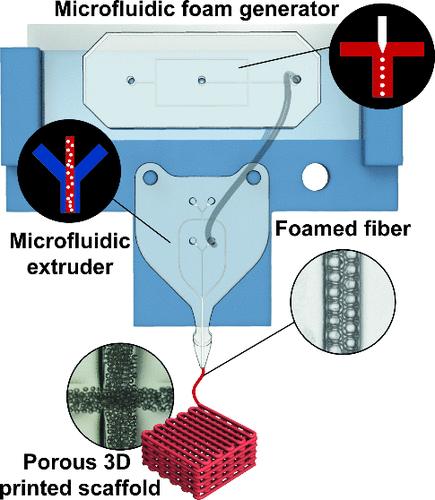
The synergistic integration of microfluidic technologies with additive manufacturing systems is advancing the development of innovative platforms to 3D bioprint scaffolds for tissue engineering with unparalleled biological relevance. Significant interest is growing in realizing porous functionally graded materials (pFGMs) that can resemble the hierarchical organization of porosity found in bone tissue. This study introduces a method for fabricating porous scaffolds based on the real-time generation of a liquid foam, which is gelled, forming porous fibers that are organized into structured matrixes using a 3D bioprinting system. The primary advantage of this approach is the possibility to adjust bubble size during printing dynamically, modifying the characteristics of the deposited foamed filaments online and in one step. As a result, locally-defined and tailor-made pores can be distributed in 3D structures with high spatial accuracy. Besides the mechanical and morphological characterization of diverse microarchitectures, we also explored the biocompatibility of the proposed approach by directly embedding osteosarcoma cells within the biomaterial. Results demonstrated the biocompatibility of the proposed methodology and revealed the influence of the interior microporosity on cell proliferation, highlighting the potential for creating tailored tissue microenvironments. The findings underscore the versatility of the presented 3D bioprinting system and its potential in fabricating biomimetic scaffolds with tailored morphological gradients, representing a substantial advancement in pFGM synthesis, with direct implications in regenerative medicine and tissue engineering.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


