A localized surface plasmon resonance effect boosts photocatalytic hydrogen evolution of ZnIn2S4/amorphous MoO3−x nanodot Z-scheme heterojunctions
IF 10.7
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Plasmonic photocatalysts have attracted considerable attention in photocatalytic systems. In this study, amorphous molybdenum oxide (MoO3−x) nanodots were anchored onto ZnIn2S4 nanospheres by a hydrothermal method. The prepared ZnIn2S4/MoO3−x (ZM) composites were then used as a visible-light-responsive catalyst to achieve photocatalytic hydrogen evolution. Afterwards, the ZM composites were extensively characterized using multiple instruments and electrochemical measurements. Afterwards, the photocatalytic activity of ZM composites was evaluated under simulated sunlight (AM 1.5G) with 10% triethanolamine (TEOA) as a sacrificial reagent. Compared to pristine ZnIn2S4, the optimal composite (ZM10) boosts the hydrogen evolution rate from 1.34 mmol g−1 h−1 to 11.08 mmol g−1 h−1 and the apparent quantum efficiency (AQE) at 420 nm from 1.81% to 6.14%. The mechanism behind the enhanced photocatalytic hydrogen evolution was explored by infrared thermal image measurement, EPR tests, XPS valence band measurement and density functional theory (DFT) calculations. The localized surface plasmon resonance (LSPR) effect of MoO3−x and the formation of an interfacial Z-scheme heterojunction are revealed to be responsible for the improved photocatalytic hydrogen evolution. Finally, the cyclic experiment results show that ZM10 maintains a high hydrogen evolution rate after 5 cycles of testing, indicating strong chemical stability and good industrial potential.
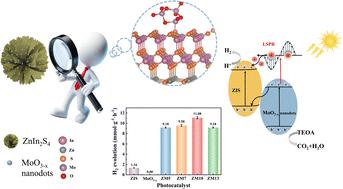
等离子体光催化剂在光催化系统中备受关注。本研究采用水热法将无定形氧化钼(MoO3-x)纳米点锚定到 ZnIn2S4 纳米球上。制备的 ZnIn2S4/MoO3-x (ZM)复合材料被用作可见光响应催化剂,以实现光催化氢气进化。随后,使用多种仪器和电化学测量方法对 ZM 复合材料进行了广泛表征。随后,以 10%的三乙醇胺(TEOA)为牺牲试剂,在模拟太阳光(AM 1.5G)下评估了 ZM 复合材料的光催化活性。与原始 ZnIn2S4 相比,最佳复合材料(ZM10)的氢气进化率从 1.34 mmol g-1 h-1 提高到 11.08 mmol g-1 h-1,420 纳米波长下的表观量子效率(AQE)从 1.81% 提高到 6.14%。通过红外热像测量、EPR 测试、XPS 价带测量和密度泛函理论(DFT)计算,探索了光催化氢进化增强的机理。结果表明,MoO3-x 的局部表面等离子体共振(LSPR)效应和界面 Z 型异质结的形成是光催化氢气进化能力增强的原因。最后,循环实验结果表明,ZM10 在经过 5 次循环测试后仍能保持较高的氢气进化率,表明其具有较强的化学稳定性和良好的工业潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


