Oxygen Nanobubbles Enhance ICG/Fe(III)-Mediated Dual-Modal Therapy To Induce Ferroptosis in Tumor Treatment
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
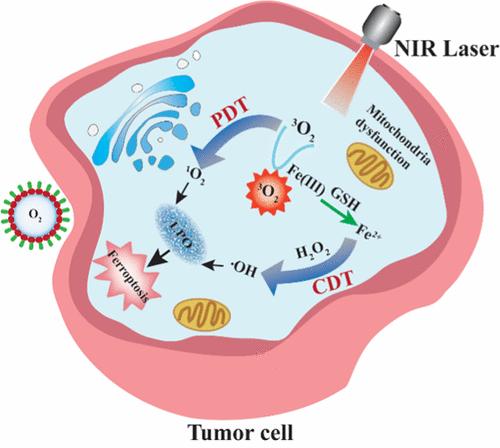
Noninvasive therapies such as photodynamic therapy (PDT) and chemodynamic therapy (CDT), which rely on reactive oxygen species (ROS), are gaining attention for their low toxicity. However, single-modal treatments have individual limitations that restrict the therapeutic efficacy. Fe(III) can coordinate with the hydrophilic regions of indocyanine green (ICG) molecules to form the ICG/Fe(III) complex, making it a promising dual-modal agent for combined PDT and CDT. However, coordination with Fe(III) leads to the aggregation quenching of ICG, hindering its application in dual-modal therapy. We innovatively utilize oxygen nanobubbles, prepared solely from water and oxygen, to significantly reverse the aggregation-induced quenching of the ICG/Fe(III) complex, thereby enhancing its stability in aqueous environments. In this system, Fe(III) assembles at the nanobubble interface, coordinating with ICG’s hydrophilic regions to form the ICG/Fe(III)-NBs. The oxygen nanobubbles boost PDT efficiency by improving the ICG/Fe(III) complex stability and oxygen content, while Fe(III) achieves CDT by generating hydroxyl radicals (•OH) through the Fenton reaction. This dual-modality treatment significantly disrupts the tumor’s redox balance, induces ferroptosis, and demonstrates strong antitumor efficacy, reducing tumor volume to 34% of its initial size in mice. The strategy offers a promising and clinically viable approach to cancer treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


