Archaean green-light environments drove the evolution of cyanobacteria’s light-harvesting system
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
Abstract
Cyanobacteria induced the great oxidation event around 2.4 billion years ago, probably triggering the rise in aerobic biodiversity. While chlorophylls are universal pigments used by all phototrophic organisms, cyanobacteria use additional pigments called phycobilins for their light-harvesting antennas—phycobilisomes—to absorb light energy at complementary wavelengths to chlorophylls. Nonetheless, an enigma persists: why did cyanobacteria need phycobilisomes? Here, we demonstrate through numerical simulations that the underwater light spectrum during the Archaean era was probably predominantly green owing to oxidized Fe(III) precipitation. The green-light environments, probably shaped by photosynthetic organisms, may have directed their own photosynthetic evolution. Genetic engineering of extant cyanobacteria, simulating past natural selection, suggests that cyanobacteria that acquired a green-specialized phycobilin called phycoerythrobilin could have flourished under green-light environments. Phylogenetic analyses indicate that the common ancestor of modern cyanobacteria embraced all key components of phycobilisomes to establish an intricate energy transfer mechanism towards chlorophylls using green light and thus gained strong selective advantage under green-light conditions. Our findings highlight the co-evolutionary relationship between oxygenic phototrophs and light environments that defined the aquatic landscape of the Archaean Earth and envision the green colour as a sign of the distinct evolutionary stage of inhabited planets. Cyanobacteria use both chlorophylls and phycobilins to absorb light energy, and authors here use cultivation experiments, numerical simulations and protein phylogenetics to argue that cyanobacteria evolved in a green-light environment during the Archaean era in which green-specialized phycobilins would have been selectively advantageous.

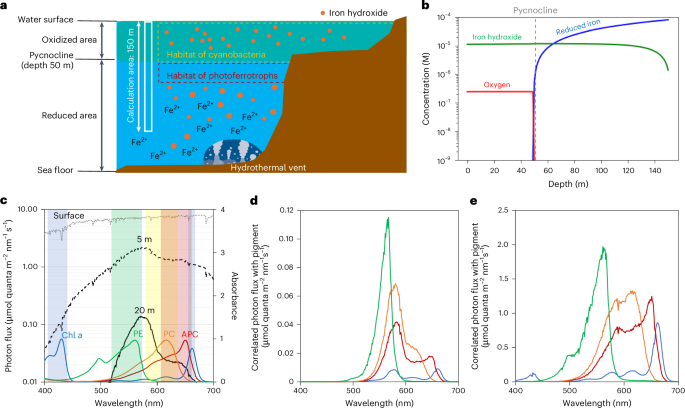
太古时代的绿光环境推动了蓝藻的光收集系统的进化
大约24亿年前,蓝藻引发了大氧化事件,可能引发了有氧生物多样性的增加。虽然叶绿素是所有光养生物普遍使用的色素,但蓝藻细菌使用称为藻胆素的额外色素作为它们的光收集天线-藻胆异体-吸收与叶绿素互补波长的光能。尽管如此,一个谜仍然存在:为什么蓝藻需要藻胆体?在这里,我们通过数值模拟证明,由于Fe(III)的氧化沉淀,太古宙时期的水下光谱可能主要是绿色的。绿光环境,可能是由光合生物塑造的,可能指导了它们自己的光合进化。对现存蓝藻进行基因工程,模拟过去的自然选择,结果表明,获得了一种叫做藻红蛋白的绿色特化藻胆素的蓝藻可以在绿光环境下繁盛。系统发育分析表明,现代蓝藻的共同祖先包含了藻胆体的所有关键成分,建立了利用绿光向叶绿素传递能量的复杂机制,因此在绿光条件下具有很强的选择优势。我们的发现强调了氧气光养生物和光环境之间的共同进化关系,这定义了太古宙地球的水生景观,并将绿色想象为有人居住的行星独特进化阶段的标志。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


