Temperature and steric hindrance-regulated selective synthesis of ketamine derivatives and 2-aryl-cycloketone-1-carboxamides via nucleophilic substitution and Favorskii rearrangement†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A selective temperature and steric hindrance-regulated method for nucleophilic substitution or Favorskii rearrangement reactions of 2-aryl-2-bromo-cycloketones with aliphatic amines has been developed to prepare ketamine derivatives and 2-aryl-cycloketone-1-carboxamides. In the presence of secondary amines or ortho-substituted 2-aryl-2-bromocycloketones, steric hindrance directs the Favorskii rearrangement to occur. Conversely, with primary amines, the product ratio of nucleophilic substitution to Favorskii rearrangement is temperature-dependent, with higher temperatures favoring the Favorskii rearrangement. At lower temperatures (−25 °C or below), nucleophilic substitution predominates, yielding ketamine derivatives in yields of 60% to 85%. This method effectively utilizes temperature and steric hindrance to control the reaction pathway and optimize product formation.
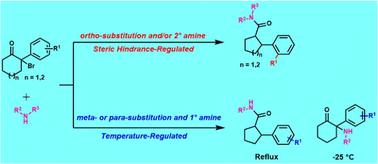
温度和空间位阻调控的氯胺酮衍生物和2-芳基环酮-1-羧酰胺的亲核取代和Favorskii重排选择性合成。
本文提出了一种选择温度和空间位阻调节的方法,通过2-芳基-2-溴环酮与脂肪胺的亲核取代或Favorskii重排反应制备氯胺酮衍生物和2-芳基-环酮-1-羧酰胺。在仲胺或邻位取代的2-芳基-2-溴环酮的存在下,空间位阻指导Favorskii重排的发生。相反,对于伯胺,亲核取代与Favorskii重排的产物比例依赖于温度,较高的温度有利于Favorskii重排。在较低的温度下(-25°C或更低),亲核取代占主导地位,氯胺酮衍生物的产率为60%至85%。该方法有效地利用温度和位阻控制反应路径,优化产物生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


