HypoxyStat, a small-molecule form of hypoxia therapy that increases oxygen-hemoglobin affinity
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
We have previously demonstrated that chronic inhaled hypoxia is remarkably therapeutic in the premier animal model of mitochondrial Leigh syndrome, the Ndufs4 knockout (KO) mouse. Subsequent work has extended this finding to additional mitochondrial diseases and more common conditions. However, challenges inherent to gas-based therapies have hindered the rapid translation of our findings to the clinic. Here, we tested a small molecule (hereafter termed HypoxyStat) that increases the binding affinity of hemoglobin for oxygen, thereby decreasing oxygen offloading to tissues. Daily oral dosing of HypoxyStat caused systemic hypoxia in mice breathing normoxic (21% O2) air. When administered prior to disease onset, this treatment dramatically extended the lifespan of Ndufs4 KO mice and rescued additional aspects of disease, including behavior, body weight, neuropathology, and body temperature. HypoxyStat was also able to reverse disease at a very late stage, thereby serving as a clinically tractable form of hypoxia therapy.
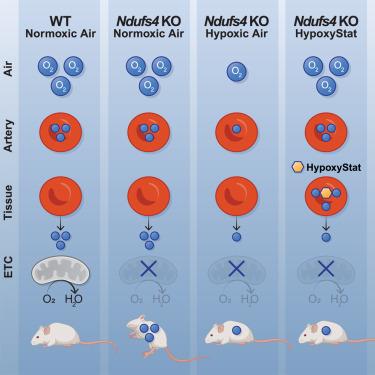
低氧,一种小分子形式的低氧治疗,增加氧血红蛋白亲和力
我们之前已经证明慢性吸入性缺氧对线粒体Leigh综合征的主要动物模型Ndufs4敲除(KO)小鼠具有显著的治疗作用。随后的工作将这一发现扩展到其他线粒体疾病和更常见的疾病。然而,气体疗法固有的挑战阻碍了我们的发现快速转化为临床。在这里,我们测试了一种小分子(以下称为HypoxyStat),它可以增加血红蛋白对氧的结合亲和力,从而减少氧向组织的卸载。每日口服HypoxyStat可引起呼吸常氧(21% O2)空气的小鼠全身性缺氧。在发病前给药,这种治疗显著延长了Ndufs4 KO小鼠的寿命,并挽救了疾病的其他方面,包括行为、体重、神经病理学和体温。HypoxyStat也能够在非常晚的阶段逆转疾病,因此作为一种临床易处理的缺氧治疗形式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


