Conformational Role of Methyl in the Potency of Cyclohexane-Substituted Squaramide CCR6 Antagonists
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
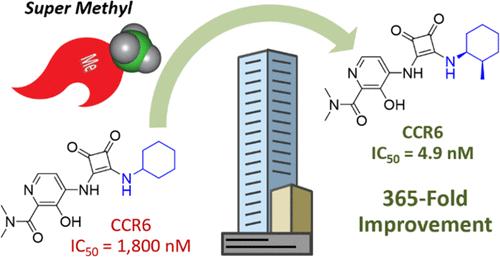
CCR6 is a chemokine receptor that mediates the migration of pathogenic inflammatory leukocytes to sites of inflammation in response to its ligand, CCL20. Herein we report the design of a potent CCR6 antagonist capable of inhibiting the chemotactic migration of CCR6+ T cells in vitro. Key to this finding was the discovery of a remarkable methyl substituent effect on antagonist potency. A 365-fold improvement in potency was observed for the cis-2-methylcyclohexanamine analogue compared to the unsubstituted cyclohexanamine derivative. Evidence generated through the characterization of conformationally restricted analogues supports the conclusion that the large potency enhancement is the result of the methyl substituent biasing the cyclohexane ring ground state conformation to favor that of the bound ligand and thus decreasing the ligand strain energy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


