Design, Synthesis, and Characterization of GluN2A Negative Allosteric Modulators Suitable for In Vivo Exploration
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
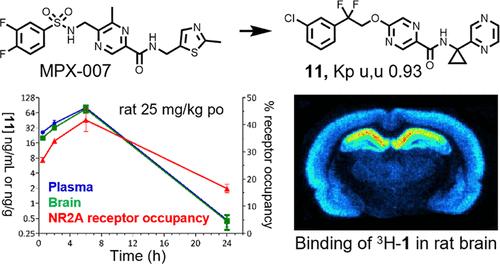
N-Methyl-d-aspartate receptors are ionotropic glutamate receptors that mediate fast excitatory neurotransmission in the central nervous system. These receptors play essential roles in synaptic plasticity, learning, and memory and are implicated in various neuropathological and psychiatric disorders. Selective modulation of NMDAR subtypes, particularly GluN2A, has proven challenging. The TCN-201 derivatives MPX-004 and MPX-007 are potent and selective for GluN2A receptors, yet their physical properties limit their in vivo utility. In this study, we optimized the MPX-004/MPX-007 scaffold by modifying the linker region between the distal halogenated aromatic ring and the central pyrazine nucleus, resulting in the identification of potent and selective compounds with improved drug-like properties. Notably, compound 1 was used to develop the first GluN2A NAM-based radioligand, and compound 11 showed improved pharmacokinetics and dose-dependent receptor occupancy in vivo. Thus, we provide an array of powerful new tools for the study of GluN2A receptors.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


