Seven-Membered Ring Formation in Triterpene Biosynthesis: A Key Cyclopropane Rearrangement in Ilelic Acid Biosynthesis
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
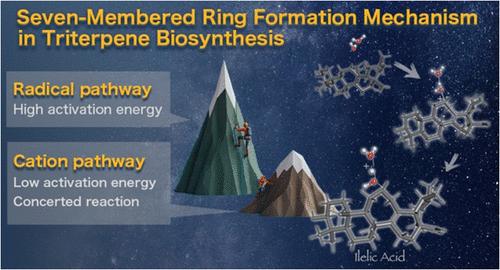
Triterpenes represent a crucial class of natural compounds with diverse biological activities and structural complexity. Among the various skeletal modifications in triterpene biosynthesis, the formation of seven-membered rings through ring expansion reactions significantly contributes to their structural diversity and, consequently, their functional versatility. This study elucidates the detailed reaction mechanism of a key seven-membered ring formation via cyclopropane rearrangement in the biosynthesis of ilelic acid. Using density functional theory (DFT) calculations, we thoroughly investigated the biosynthetic pathway of ilelic acid, focusing on the critical ring expansion step. Our computational analysis reveals that the seven-membered ring formation proceeds through a cationic mechanism rather than a radical-mediated process. Notably, we found that the inherent instability of the secondary carbocation intermediate drives a concerted reaction pathway, avoiding the formation of high-energy intermediates. This mechanistic understanding not only sheds light on the biosynthesis of ilelic acid but also offers broader implications for comprehending similar transformations in other triterpene biosynthetic pathways. Our findings contribute to the fundamental understanding of triterpene skeletal diversity and pave the way for potential biomimetic approaches in the synthesis of complex seven-membered ring-containing terpenes. Furthermore, this work underscores the power of computational methods in unraveling intricate biosynthetic mechanisms.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


