H4K12 lactylation potentiates mitochondrial oxidative stress via the Foxo1 pathway in diabetes-induced cognitive impairment
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Aims
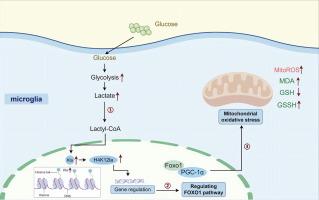
To investigate the role and potential mechanisms of H4K12 lactylation modifications in diabetes-related cognitive impairment (DACD).Methods
Behavioral tests, HE staining, and immunohistochemistry were employed to assess cognitive function and the extent of brain tissue injury. Metabolomics and proteomics were applied to profile the metabolic regulatory network. We measured lactic acid and Pan-Kla levels in the brains of T2DM mice and high glucose-treated microglia. CUT&Tag technology was utilized to identify genes regulated by H4K12la. Small interfering RNA (siRNA) sequences and adeno-associated viruses (AAVs) were used to knock down key components in signaling pathways, evaluating the impact of histone lactylation on microglial polarization.Results
Lactic acid levels were significantly higher in the brains of T2DM mice and high glucose-treated microglia compared to controls, leading to an increase in pan histone lysine lactylation (Kla). We found that lactate directly induced an increase in H4K12la. CUT&Tag analysis revealed that elevated H4K12la activates the FOXO1/PGC-1α signaling pathway by enhancing binding to the FOXO1 promoter, promoting mitochondrial oxidative stress.Conclusion
This study demonstrated that elevated H4K12la directly activates the FOXO1 signaling pathway, promoting oxidative stress and contributing to DACD phenotypes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


