Impact of Fluorine Pattern on Lipophilicity and Acid–Base Properties of 2-(Thiofluoroalkyl)pyridines: Insights from Experiments and Statistical Modeling
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
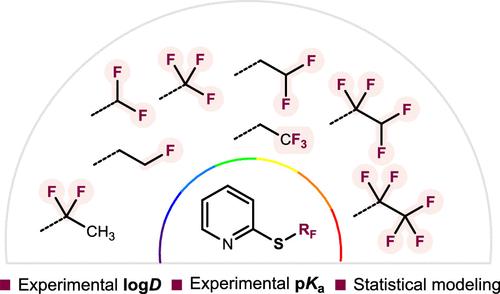
Lipophilicity and acid–base properties are two key aspects of the optimization of a compound in drug discovery. Using 19F NMR, we experimentally determined the log D7.4 of a wide array of 2-thiofluoroalkyl (SRF) and 2-sulfonyl fluoroalkyl (SO2RF) substituted pyridines and the pKa values of their protonated counterparts. Statistical modeling based on constitutional and DFT descriptors provided further insights into the structure–property relationship, explaining the experimental observations and predicting log D7.4 values. Our results highlight the influence of fluorination topology in SRF fragments and demonstrate the dual effect of fluorine on molecular polarity, increasing the hydrophobic surface and the polarity of the sulfur moiety. By analyzing methyl- and ethyl-derived fragments, we found a gradient in log D7.4 values influenced by the oxidation state of the sulfur atom and fluorination pattern. Our findings emphasize the context-dependent impact of fluorination and offer insights to better understand the impact of thiofluoroalkyl chains on these physicochemical properties.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


