Design, Synthesis, and Biological Evaluation of BODIPY-Caged Resiquimod as a Dual-Acting Phototherapeutic
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
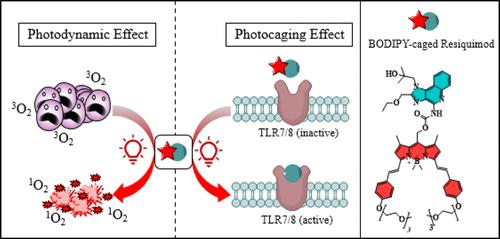
Resiquimod, an imidazoquinoline scaffold, exhibits potent immunotherapeutic activity but is associated with off-target effects, limiting its clinical utility. To address this limitation, we developed a novel BODIPY-caged resiquimod that is responsive to red light, combining photocaging and photodynamic therapy functionalities. Molecular docking studies guided identification of the optimal caging site for resiquimod, effectively masking its immune activity. BODIPY-caged resiquimod remained inactive under dark conditions, effectively masking resiquimod’s immunostimulatory effects. However, red light irradiation precisely uncaged resiquimod, inducing robust immune activation, even in the presence of N-acetyl cysteine as an antioxidant. Notably, the attachment of resiquimod to BODIPY reduced the dark toxicity typically associated with BODIPY as a photosensitizer. In 3D spheroid models of HeLa and A549 cells, BODIPY-caged resiquimod demonstrated spatiotemporal control over cytotoxicity, significantly enhancing cell death only upon irradiation. This dual-function therapeutic approach highlights a “win–win” strategy: precise, red-light-mediated control of immune activation and photodynamic efficacy with reduced collateral toxicity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


