Injectable Chondroitin Sulfate Microspheres with Gallic Acid-Magnesium MOF for Anti-Inflammatory and Cartilage Degeneration Alleviation in Osteoarthritis Treatment
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
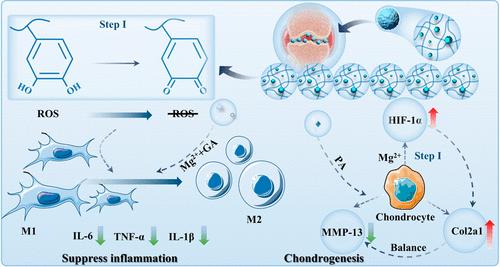
Inflammation and cartilage degeneration are critical challenges in osteoarthritis (OA) treatment. Achieving sustained drug efficacy while mitigating the adverse effects of inflammation and reactive oxygen species remains a significant challenge. This study synthesizes a gallic acid-magnesium (GA-Mg) metal–organic framework (MOF) as a drug carrier for puerarin (PA). The PA-loaded GA-Mg MOF (pGM) is encapsulated within chondroitin sulfate methacrylate, forming monodisperse hybrid microspheres (CM@pGM) under ultraviolet light using microfluidic technology. The pGM is physically confined within the microspheres through a network of structural obstructions and noncovalent interactions. During degradation, GA and Mg2+ ions release from pGM, improving the inflammatory microenvironment of the articular cavity and mitigating oxidative stress. The sustained release of Mg2+ and PA supports chondrocyte anabolism and facilitates cartilage repair. In vitro studies confirm that injectable microspheres extend the drug release period to over 2 weeks. In vivo experiments demonstrate that CM@pGM significantly reduces osteophyte formation, alleviates degenerative changes in articular cartilage, and delays OA progression. In conclusion, CM@pGM, as a drug delivery platform that ameliorates the inflammatory microenvironment, alleviates oxidative stress, and promotes cartilage repair, holds significant potential for OA treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


