Nitrogen vacancies cobalt nitride and its loading Pt electrocatalysts for efficient overall water splitting
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
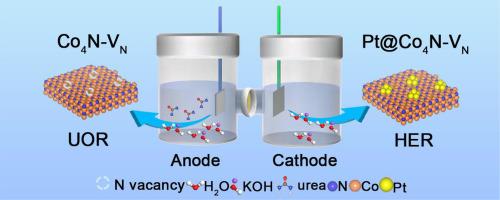
Anion exchange membrane electrolysis (AEMWE) is currently a promising technology to produce hydrogen from water. Developing the highly intrinsic activity of electrodes is extremely important. In this paper, nitrogen vacancies-rich cobalt nitride (Co4N-VN) is used as the anode catalyst of the urea oxidation reaction (UOR) and its loading Pt (Pt@Co4N-VN) acted as the cathode of hydrogen evolution reaction (HER). The introduction of N-vacancies gained the electron-deficient Co4N more favorable for the UOR process. Meanwhile, the electron transfer between the Co4N-VN carrier and Pt can enhance the intrinsic HER activity of loaded Pt. Specifically, in the 0.33 M urea and 1.0 M KOH electrolyte, the UOR potential of CO4N-VN is only 1.58 V at the current density of 300.0 mA cm−2, which is much lower than that of Co4N and Co3O4. At the same time, the HER overpotential at 1.0 M KOH and 300.0 mA cm−2 is only 120.0 mV, lower than 20 wt% Pt/C. By measuring the bode phase diagram, the presence of N-vacancies can accelerate the electron transfer rate of catalysts to improve the UOR and HER electrocatalytic activity. The overall water-splitting device featuring Pt@Co4N-VN||Co4N-VN electrodes achieves a voltage of 2.99 V at a current density of 300.0 mA cm−2.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


