Dissecting Structural Requirements of Leucinostatin A Derivatives for Antiprotozoal Activity and Mammalian Toxicity
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
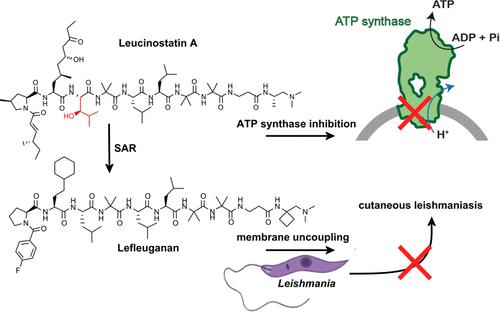
Lefleuganan, a clinical stage drug candidate for the treatment of cutaneous leishmaniasis, is a synthetic nonapeptide inspired by the natural antimicrobial peptide leucinostatin A, exhibiting excellent antiprotozoal activity in the low nM range. Lefleuganan shows strongly reduced acute toxicity, making it amenable for clinical use. Here, using a broad set of in vivo and in vitro measurements using purified enzymes, we find that leucinostatin A, but not lefleuganan, specifically targets the mitochondrial ATP synthase, inhibiting ATP synthesis by the human, bovine, and yeast enzyme in the nanomolar range. In a structure–activity relationship study covering the chemical space between the two compounds, hydroxyleucine at position 7 in leucinostatin A is identified as the key responsible moiety for specific ATP synthase inhibition and systemic toxicity. Our data suggest that efficient antiprotozoal activity of these class of compounds is mediated by efficient energetic uncoupling of negatively charged membranes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


