Near-Infrared Long-Lived Luminescent Nanoparticle-Based Time-Gated Imaging for Background-Free Detection of Avian Influenza Virus
IF 8.2
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
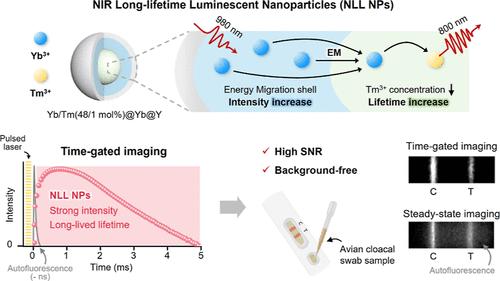
Near-infrared (NIR)-to-NIR upconversion nanoparticles (UCNPs) are promising materials for biomedical imaging and sensing applications. However, UCNPs with long lifetimes continue to face the limitation that they are usually accompanied by weak luminescence intensity, resulting in difficulties in achieving high-resolution and sensitive time-gated imaging. To overcome this limitation, we have developed NIR long-lifetime luminescent nanoparticles (NLL NPs) with strong 800 nm emission by adding a photosensitizing shell and with a prolonged lifetime by lowering the activator concentration. NLL NP-based time-gated imaging overcomes the inherent limitations of steady-state imaging by providing higher signal-to-noise ratios and more robust signal intensities. When integrated into a lateral flow immunoassay (LFA) for the detection of avian influenza viruses, NLL NP-based time-gated imaging demonstrates a 32-fold lower limit of detection compared to conventional optimal 800 nm emitting nanoparticles. Furthermore, the high accuracy of the NLL NP-based LFA is confirmed through clinical validations using 65 samples, achieving a sensitivity and specificity of 100% and an area under the curve of 1.000. These results demonstrate the potential of NLL NP-based time-gated imaging as a powerful tool for the highly sensitive and accurate detection of avian influenza viruses in complex clinical samples.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sensors
Chemical Engineering-Bioengineering
CiteScore
14.50
自引率
3.40%
发文量
372
期刊介绍:
ACS Sensors is a peer-reviewed research journal that focuses on the dissemination of new and original knowledge in the field of sensor science, particularly those that selectively sense chemical or biological species or processes. The journal covers a broad range of topics, including but not limited to biosensors, chemical sensors, gas sensors, intracellular sensors, single molecule sensors, cell chips, and microfluidic devices. It aims to publish articles that address conceptual advances in sensing technology applicable to various types of analytes or application papers that report on the use of existing sensing concepts in new ways or for new analytes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


