Design and synthesis of novel thioether analogs as promising antiviral agents: In vitro activity against enteroviruses of interest
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
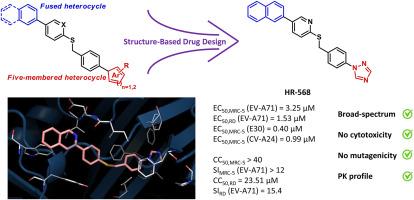
The Enterovirus genus contains two major subgroups: rhinovirus (RV) species A-C and enterovirus (EV) ones A-D. While RV only infects the respiratory system, the EV can cause a wide variety of diseases, ranging from non-specific febrile illness to severe neurologic complications. To date, no curative treatments are commercially available. Our research team had recently developed EV-A71 inhibitors. To improve their activity and broaden their spectrum, we performed optimization of the structure following an iterative cycle of chemical modulations. As a result, we obtained two broad-spectrum inhibitors with micromolar activity against these 3 types of viruses (OM1260: EC50 (MRC-5, EV-A71) = 1.15 μM; EC50 (RD, EV-A71) = 4.38 μM; EC50 (MRC-5, E30) = 0.41 μM; EC50 (MRC-5, CVA24) = 1.15 μM; HR-568: EC50 (MRC-5, EV-A71) = 3.25 μM; EC50 (RD, EV-A71) = 1.53 μM; EC50 (MRC-5, E30) = 0.40 μM; EC50 (MRC-5, CVA24) = 1.22 μM). Docking studies shed light on structure-activity relationships, while time-of-drug addition assays confirmed their intervention during the early step of viral replication. Eventually, some pharmacokinetic modelling has been carried out to evaluate their druggability. All these results showed that OM1260 and HR-568 are promising candidates for further development.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


