Discovery of 3-hydroxypyridin-4(1H)-ones ester of ciprofloxacin as prodrug to combat biofilm-associated Pseudomonas aeruginosa
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
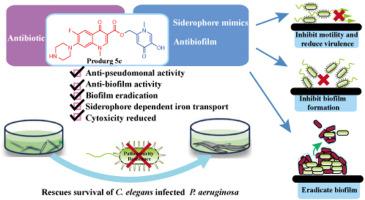
Chronic infections by Pseudomonas aeruginosa (P. aeruginosa) are frequently complicated due to its ability to form biofilm, which also effectively enhance its resistance to antibiotics. Bacteria-specific antibiotic delivery could locally increase drug concentration to break antimicrobial resistance and reduce the drug's peripheral side effects. The standard-of-care drug ciprofloxacin suffers from severe systemic side effects and was therefore chosen for this approach. It has been identified that 3-hydroxypyridin-4(1H)-one as siderophore mimics could be utilized by P. aeruginosa, and reduced bacterial biofilm formation. In this work, ciprofloxacin was conjugated to 3-hydroxypyridin-4(1H)-one by cleavable linkers to yield prodrugs, which were strategically designed and synthesized to function as dual antibacterial and antibiofilm agents against P. aeruginosa. Conjugate 5c was identified and has the best minimum inhibitory concentrations of 1.07 μM against P. aeruginosa PAO1, and reduced 61.7 % of biofilm formation. In addition, 5c destroyed 75.7 % of mature biofilms. Further studies on the uptake mechanisms showed that the bacterial siderophore-dependent iron transport system was involved in the uptake of the conjugates. Conjugate 5c interfered with iron uptake by bacteria, inhibited their motilities and reduced the production of virulence. Furthermore, prodrug 5c reduced toxicity in vivo and in vitro and showed a positive therapeutic effect in the treatment of Caenorhabditis elegans (C. elegans) infected by P. aeruginosa. These results demonstrate that 3-hydroxypyridin-4(1H)-ones-ciprofloxacin prodrugs are potent in the treatment of biofilm-associated drug-resistant P. aeruginosa infections.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


