Comprehensive single-cell analysis deciphered the immunoregulatory mechanism of TPPU in alleviating sepsis-related acute liver injury
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Sepsis-related acute liver injury involves complex immune dysfunctions. Epoxyeicosatrienoic acids (EETs), bioactive molecules derived from arachidonic acid (AA) via cytochrome P450 (CYP450) and rapidly hydrolyzed by soluble epoxide hydrolase (sEH), possess anti-inflammatory properties. Nevertheless, the impact of the sEH inhibitor TPPU on sepsis-related acute liver injury remains uncertain.
Objectives
This study utilized comprehensive single-cell analysis to investigate the immunoregulatory mechanism of TPPU in alleviating sepsis-related acute liver injury.
Methods
Hepatic bulk RNA sequencing and proteomics analyses were employed to investigate the mechanisms underlying sepsis-related acute liver injury induced by cecal ligation and puncture in mice. Cytometry by time-of-flight and single-cell RNA sequencing were conducted to thoroughly examine the immunoregulatory role of TPPU at single-cell resolution.
Results
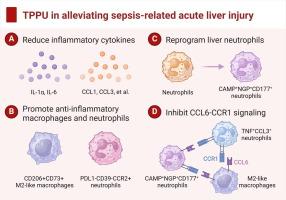
Downregulation of AA metabolism and the CYP450 pathway was observed during sepsis-related acute liver injury, and TPPU treatment reduced inflammatory cytokine production and mitigated sepsis-related hepatic inflammatory injury. Comprehensive single-cell analysis revealed that TPPU promotes the expansion of anti-inflammatory CD206+CD73+ M2-like macrophages and PDL1-CD39-CCR2+ neutrophils, reprogramming liver neutrophils to an anti-inflammatory CAMP+NGP+CD177+ phenotype. Additionally, TPPU inhibits the CCL6-CCR1 signaling mediated by M2-like macrophages and CAMP+NGP+CD177+ neutrophils, altering intercellular communication within the septic liver immune microenvironment.
Conclusion
This study demonstrated TPPU’s protective efficacy against sepsis-related acute liver injury, underscoring its vital role in modulating liver macrophages and neutrophils and enhancing prospects for personalized immunomodulatory therapy.

综合单细胞分析揭示了TPPU减轻脓毒症相关急性肝损伤的免疫调节机制
败血症相关性急性肝损伤涉及复杂的免疫功能障碍。环氧二碳三烯酸(EETs)是一种由花生四烯酸(AA)经细胞色素P450 (CYP450)提取并被可溶性环氧化物水解酶(sEH)快速水解的活性分子,具有抗炎作用。然而,sEH抑制剂TPPU对脓毒症相关急性肝损伤的影响仍不确定。目的通过单细胞综合分析,探讨TPPU减轻脓毒症相关急性肝损伤的免疫调节机制。方法采用肝大体积RNA测序和蛋白质组学分析,探讨盲肠结扎穿刺致小鼠败血症相关性急性肝损伤的机制。通过飞行时间和单细胞RNA测序的细胞术,在单细胞分辨率下彻底检查TPPU的免疫调节作用。结果脓毒症相关急性肝损伤中AA代谢和CYP450通路下调,TPPU治疗可减少炎症细胞因子的产生,减轻脓毒症相关肝脏炎症损伤。综合单细胞分析显示,TPPU促进抗炎CD206+CD73+ m2样巨噬细胞和PDL1-CD39-CCR2+中性粒细胞的扩增,将肝中性粒细胞重编程为抗炎CAMP+NGP+CD177+表型。此外,TPPU抑制由m2样巨噬细胞和CAMP+NGP+CD177+中性粒细胞介导的CCL6-CCR1信号传导,改变脓毒肝免疫微环境中的细胞间通讯。结论TPPU对脓毒症相关急性肝损伤具有保护作用,在调节肝脏巨噬细胞和中性粒细胞中发挥重要作用,为个性化免疫调节治疗提供了前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


