Synergistic Metal-Support Interactions in Au/GaN Catalysts for Photoelectrochemical Nitrate Reduction to Ammonia
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Metal-support interactions are crucial in the electrochemical synthesis of ammonia (NH3) from nitrate (NO3−) reduction reaction, enabling efficient NH3 production under mild conditions. However, the complexity of the reaction pathways often limits efficiency. Here, a photoelectrochemical system composed of gold (Au) nanoclusters supported on gallium nitride (GaN) nanowires is introduced, grown on a n+-p Si wafer, for selective reduction of NO3− to NH3 under solar illumination. NO3− ions are preferentially adsorbed and reduced to nitrite (NO2−) on the GaN nanowires, which then transfer to adjacent Au nanoclusters to complete the NH3 synthesis. This mechanism is confirmed by both experimental data and theoretical calculations. Optimizing the surface coverage and size of Au nanoclusters on the GaN nanowires significantly enhanced catalytic activity compared to that on planar n+-p Si photoelectrodes, achieving a faradaic efficiency of 91.8% at −0.4 VRHE and a high NH3 production rate of 131.1 µmol cm−2 h−1 at −0.8 VRHE. These findings highlight the synergetic effect between metal co-catalysts and semiconductor supports in designing photoelectrodes for multi-step NO3− reduction.
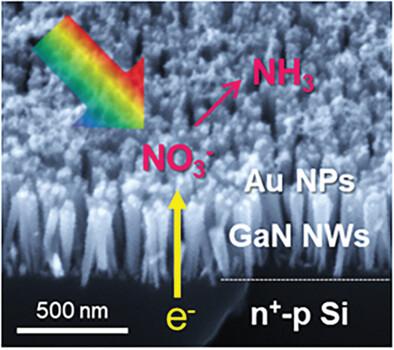
在硝酸盐(NO3-)还原反应电化学合成氨(NH3)的过程中,金属支架之间的相互作用至关重要,可在温和条件下高效生产 NH3。然而,反应途径的复杂性往往限制了效率。在此,我们介绍了一种由氮化镓(GaN)纳米线上支撑的金(Au)纳米团簇组成的光电化学系统,该系统生长在 n+-p 硅晶片上,可在太阳光照射下将 NO3- 选择性还原为 NH3。氮氧化物离子在氮化镓纳米线上优先被吸附并还原成亚硝酸盐(NO2-),然后转移到相邻的金纳米团簇上完成 NH3 的合成。实验数据和理论计算都证实了这一机制。与平面 n+-p 硅光电极相比,优化 GaN 纳米线上金纳米团簇的表面覆盖率和尺寸可显著提高催化活性,在 -0.4 VRHE 条件下,催化效率达到 91.8%,在 -0.8 VRHE 条件下,NH3 生成率高达 131.1 µmol cm-2 h-1。这些发现凸显了金属助催化剂和半导体支持物在设计用于多步还原 NO3- 的光电极方面的协同效应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


