Origin and stepwise evolution of vertebrate lungs
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
Abstract
Lungs are essential respiratory organs in terrestrial vertebrates, present in most bony fishes but absent in cartilaginous fishes, making them an ideal model for studying organ evolution. Here we analysed single-cell RNA sequencing data from adult and developing lungs across vertebrate species, revealing significant similarities in cell composition, developmental trajectories and gene expression patterns. Surprisingly, a large proportion of lung-related genes, coexpression patterns and many lung enhancers are present in cartilaginous fishes despite their lack of lungs, suggesting that a substantial genetic foundation for lung development existed in the last common ancestor of jawed vertebrates. In addition, the 1,040 enhancers that emerged since the last common ancestor of bony fishes probably contain lung-specific elements that led to the development of lungs. We further identified alveolar type 1 cells as a mammal-specific alveolar cell type, along with several mammal-specific genes, including ager and sfta2, that are highly expressed in lungs. Functional validation showed that deletion of sfta2 in mice leads to severe respiratory defects, highlighting its critical role in mammalian lung features. Our study provides comprehensive insights into the evolution of vertebrate lungs, demonstrating how both regulatory network modifications and the emergence of new genes have shaped lung development and specialization across species. An analysis of single-cell RNA sequencing data from adult and developing lungs across vertebrate species reveals genetic components in the common ancestor of jawed vertebrates and new lung-specific enhancers that contribute to the evolution of vertebrate lungs.

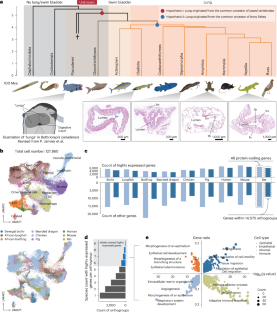
脊椎动物肺的起源和逐步进化
肺是陆生脊椎动物必不可少的呼吸器官,在大多数硬骨鱼类中存在,但在软骨鱼类中不存在,使其成为研究器官进化的理想模型。在这里,我们分析了脊椎动物成年和发育中肺的单细胞RNA测序数据,揭示了细胞组成、发育轨迹和基因表达模式的显著相似性。令人惊讶的是,尽管软骨鱼类没有肺,但它们却存在很大比例的肺相关基因、共表达模式和许多肺增强因子,这表明在有颌脊椎动物的最后共同祖先中存在肺发育的大量遗传基础。此外,自硬骨鱼类的最后一个共同祖先以来出现的1040个增强子可能含有导致肺部发育的肺特异性元素。我们进一步确定肺泡1型细胞是哺乳动物特有的肺泡细胞类型,以及几种哺乳动物特有的基因,包括ager和sfta2,在肺中高度表达。功能验证表明,小鼠中sfta2的缺失会导致严重的呼吸缺陷,突出了其在哺乳动物肺部特征中的关键作用。我们的研究为脊椎动物肺的进化提供了全面的见解,展示了调节网络的修改和新基因的出现如何影响了物种间肺的发育和特化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


