Impact of sodium poly(4-styrene sulfonate) coating on the dissolution kinetics of fluorite nanoparticles
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
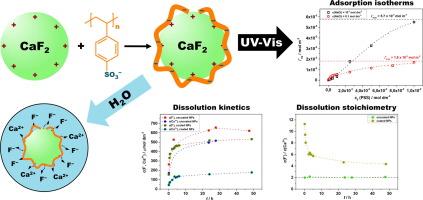
The adsorption of poly(4-styrene sulfonate) (PSS) polyanions onto the positively charged surface of fluorite nanoparticles, along with the dissolution of fluorite, was investigated using UV–Vis spectrophotometry, dynamic light scattering, and electrophoresis. Optimal conditions for adsorption were established, and the dissolution kinetics of fluorite nanoparticles in water at different pH values were analysed both before and after coating with PSS, using fluoride (F-ISE) and calcium ion-selective electrodes (Ca-ISE). Dynamic light scattering and electrophoretic measurements were employed to assess particle size and electrokinetic potential during dissolution. During the study of dissolution kinetics Ca-ISE data show a decreasing dissolution rate with increasing pH. Dissolution of coated particles was generally slower, with Ca-ISE data indicating the release of calcium ions from the fluorite surface and the substitution of sodium ions bound to the polyelectrolyte coatings. This initial stimulation of fluorite dissolution diminishes over time due to the slower diffusion of dissolved ions through the polyelectrolyte coating.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


