Benzannulation of Functionally Enhanced Indole-3-carbaldehydes with Ynones and Alkynoates: A Domino Approach to Bioactive Carbazoles─Synthesis of Clauolenzole A, Calothrixin A & B, Methyl Carbazole-3-carboxylate, and Quinocarbazole
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-05
DOI:10.1021/acs.joc.4c0319110.1021/acs.joc.4c03191
引用次数: 0
Abstract
A flexible, regioselective, benzannulation strategy toward multifunctional carbazoles from 2-(2-oxo-2-arylethyl)indole-3-carbaldehydes, employing either ynones or alkynoates as reaction partners, has been envisaged and implemented. This enabling access to variegated carbazoles in one-flask operation leads to strategic substituent diversification via reaction partner variation. The efficacy and applications of this methodology are demonstrated through 23 examples and concise syntheses of bioactive clauolenzole A, calothrixin A & B, methyl carbazole-3-carboxylate, and pharmacophoric quinocarbazole.
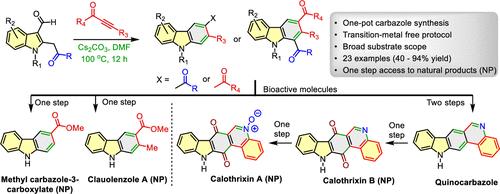
吲哚-3-氨基甲酸酯与炔酮和炔诺酸酯的功能增强联苯:生物活性咔唑的多米诺骨牌法──clololenzole A、Calothrixin A和B、甲基咔唑-3-羧酸酯和喹咔唑的合成
一个灵活的,区域选择性的,苯并环化策略从2-(2-氧-2-芳基乙基)吲哚-3-乙醛合成多功能咔唑,采用炔酮或炔酸盐作为反应伙伴,已经被设想和实施。这使得在一个烧瓶操作中获得多样化的咔唑通过反应伙伴的变化导致战略性取代基多样化。通过23个例子和简明合成生物活性clolenzole A, calothrixin A &;B,甲基咔唑-3-羧酸酯,和药效喹啉咔唑。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


