Development of Ferrocenyl and Ruthenocenyl Zileuton Analogs with Enhanced Bioactivity toward Human 5-Lipoxygenase: Innovation in Drugs for Inflammatory Diseases
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
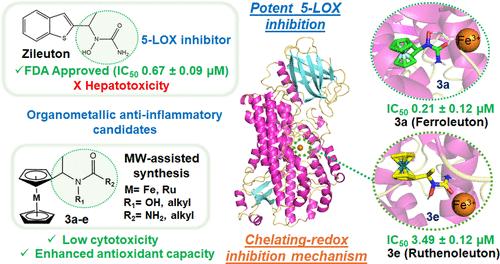
Zileuton is the only FDA-approved 5-lipoxygenase (5-LOX) inhibitor for asthma treatment, but it produces hepatotoxicity associated with the benzothiophene fragment. Using the concept of organometallic derivatization pioneered by Jaouen and Brocard, we synthesized five new organometallic Zileuton derivatives, maintaining the urea fragment and incorporating ferrocenyl and ruthenocenyl moiety (3a–e). Their biological activity was evaluated against 5-LOX, 15-LOX, COX-1, and COX-2 enzymes. The ferrocenyl and ruthenocenyl N-hydroxyurea complexes coined Ferroleuton (3a) and Ruthenoleuton (3e) showed the highest selective inhibitory activity against 5-LOX, with IC50 values of 0.21 ± 0.12 and 3.49 ± 1.11 μM, respectively. Notably, 3a exhibited superior activity compared to Zileuton (IC50 0.67 ± 0.09 μM), demonstrating the key role of N-hydroxyurea and ferrocenyl fragments in the inhibitory process. Worthy of note, both compounds displayed low cytotoxicity in lung fibroblast healthy cells line (MRC-5) (CC50 of 116.40 and >200 μM, respectively). Enzyme kinetic studies indicated competitive and mixed types of inhibition for 3a and 3e, respectively. Additionally, they demonstrated superior antioxidant capacity compared to Zileuton (DPPH, ABTS, and FRAP assays). Electrochemical and molecular dynamics (MD) studies suggest a chelating-redox deactivation mechanism for 5-LOX. These findings position Ferroleuton (3a) and Ruthenoleuton (3e) as promising candidates for inflammatory disease treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


