Solvent Extraction and Coordination Properties of 1,10-Phenanthroline-2,9-dicarboxylic Acid Diamides with Alkyl-Aryl Substituents toward Lanthanides(III) and Americium(III)
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
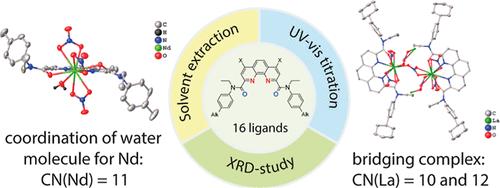
For a wide range of 1,10-phenanthroline-2,9-dicarboxylic acid diamides (DAPhen), differing in the structure of the amide substituent and the presence of chlorine atoms in the 4,7-positions of the phenanthroline core, the extraction properties for lanthanides(III) and americium(III) were studied. Protonation and binding constants to Eu(III) were determined by using UV–vis titration. The values of binding constants and protonation constants are higher for nonsubstituted diamides than for 4,7-chlorinated diamides, which is in good agreement with the solvent extraction data. The structures of more than 20 complexes with lanthanide nitrates in the solid state have been determined using X-ray diffraction analysis. Unusual DAPhen ligand complexes with lanthanum and neodymium nitrates were obtained. For the first time, the possibility of entering a water molecule into the internal coordination sphere of neodymium in a complex with the DAPhen ligand was shown, resulting in the coordination number of neodymium increasing to 11. An unusual complex with a bridge structure was obtained for lanthanum nitrate in which the lanthanum ions have different coordination numbers of 10 and 12.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


